QSAR AND DRUG DESIGN Corwen Hansch developed a






































- Slides: 79

QSAR AND DRUG DESIGN

Corwen Hansch developed a workable methodology Quantitative Structure Activity Relationship. In 1968, Crum-Brown and Fraser published an equation first general formulation of QSAR’S.

¡ The relationship b/w chemical structure and biological activity since before the turn of the century.

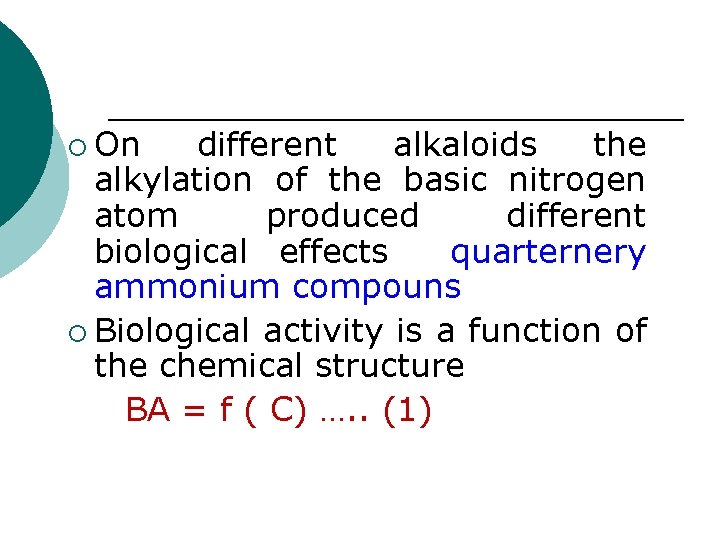
¡ On different alkaloids the alkylation of the basic nitrogen atom produced different biological effects quarternery ammonium compouns ¡ Biological activity is a function of the chemical structure BA = f ( C) …. . (1)

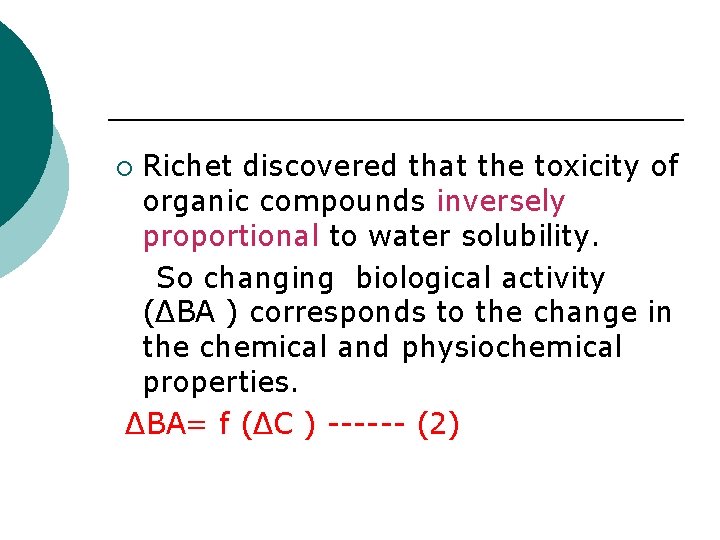
Richet discovered that the toxicity of organic compounds inversely proportional to water solubility. So changing biological activity (ΔBA ) corresponds to the change in the chemical and physiochemical properties. ΔBA= f (ΔC ) ------ (2) ¡

¡ ¡ All the QSAR equation corresponds to eqn 2, because only difference in BA quantitatively correlates with changes in lipophilicity of the compound under investigation. QSAR involves the derivation of mathematical formula which relates the biological activities of a group of compounds to their measurable physiochemical parameters.

These parameters have major influence on drug’s activity. Biological activity= function(parameters) Activity is expressed as log(1/c), c is the minimum concentration required to cause a defined biological response. QSAR based on Hammet’s constant uses eletronic properties as the descriptors of structures. ¡

The importance of lipophilicity expressed as octanol-water partition coefficient on biological activity. ¡ This parameter provides a measure of bioavailability to compounds which will determine the amt of compounds that reaches the target site. ¡

All these reveal that biological activity of a drug is a function of chemicalfeatures(lipophilicity, electro nic and steric) of the substituents and skeleton of the molecule. ¡ Lipophilicity is the major factor governing transport, distribution and metabolism of the drug in biological system. ¡

Electronic and steric features influence the metabolism and pharmacodynamic process of the drug. ¡ A major problem in QSAR studies arise because hydrophobic, electronic and steric effect overlaps and cannot be separated. ¡

Parameters assigned to various chemical grps that can be used to modify the structure of a drug. ¡ The parameter is a measure of the potential contribution of chemical group to a particular property of the parent drug. ¡

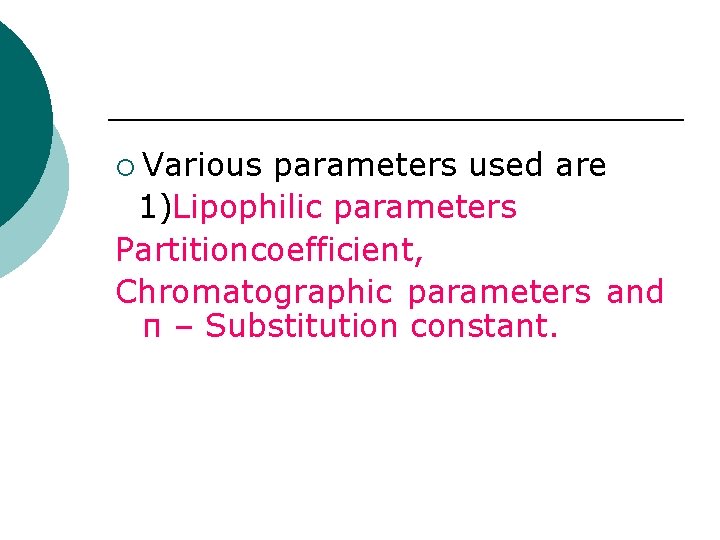
¡ Various parameters used are 1)Lipophilic parameters Partitioncoefficient, Chromatographic parameters and π – Substitution constant.

ii)Polarizability parameters: Molar refractivity(MR), Molarvolume, Parachor. iii)Electronic parameters: Hammett Constant, Field and resonance parameters, parameters derived from spectroscopic data, Charge-transfer constant, Dipole moment, Quantum chemical parameters. ¡

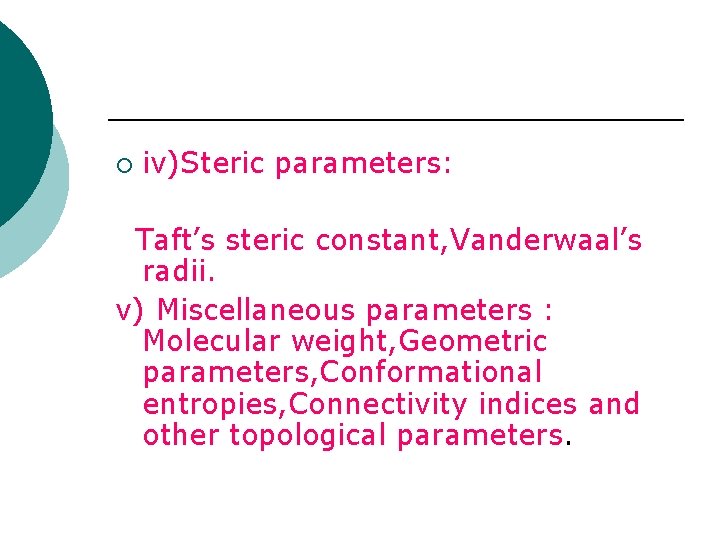
¡ iv)Steric parameters: Taft’s steric constant, Vanderwaal’s radii. v) Miscellaneous parameters : Molecular weight, Geometric parameters, Conformational entropies, Connectivity indices and other topological parameters.

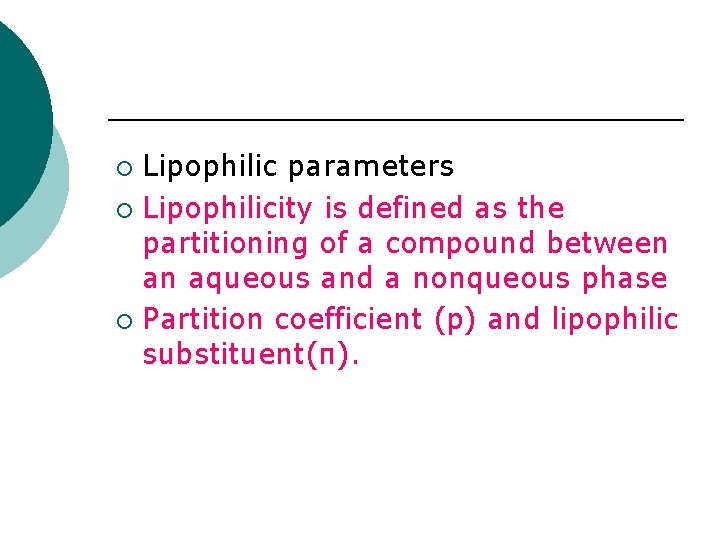
Lipophilic parameters ¡ Lipophilicity is defined as the partitioning of a compound between an aqueous and a nonqueous phase ¡ Partition coefficient (p) and lipophilic substituent(π). ¡

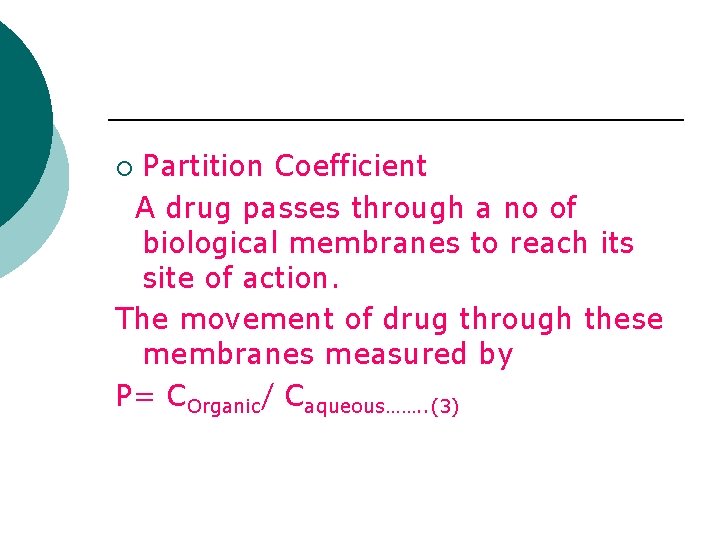
Partition Coefficient A drug passes through a no of biological membranes to reach its site of action. The movement of drug through these membranes measured by P= COrganic/ Caqueous……. . (3) ¡

¡ It is the ratio of concentration of the substance in organic and aqueous phase of a two compartment system under equillibrium conditions. For easily ionisable drug P= COrganic/ Caqueous(1 -α ) Alpha is the degree of ionisation.

The acuracy of correlation of drug activity wit coefficient will depend on the solvent system used as a model for the membrane. ¡ Both pure and buffered soln used for the aqueous soln. The n-octanol water system is frequently chosen, because it appears to be a good mimic of lipid polarity. ¡

n-octanol more constistent results for drugs absorbed in GI tract less polar solvents such as olive oil give more consistent correlation of drugs crossing the blood brain barrier. ¡

n-octanol has a low vapour pressure, allowing reproducible measurements. ¡ N-octanol is a UV transaparent over a large range making the quantitative detemination of a compound very easy. ¡

More polar solvents such as chloroform gives more consistent value for buccal absorption. ¡ It is a suitable model of the lipid constituents of biological membrane due to its long alkyl chain and the polar hydroxyl grp. ¡

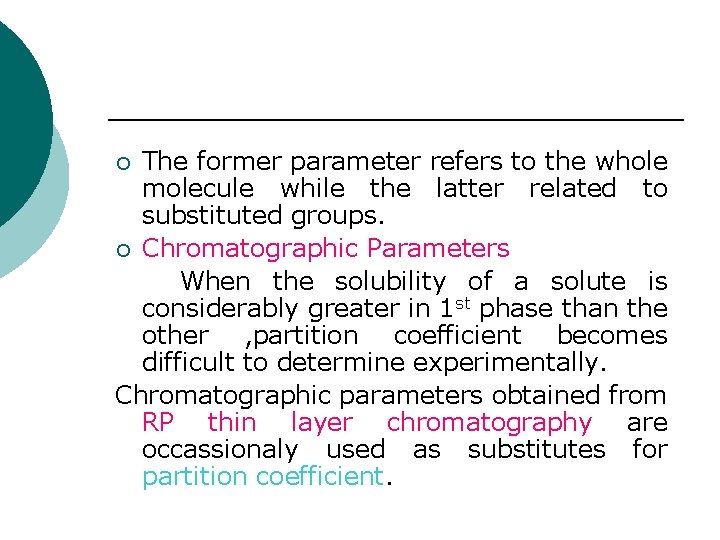
The former parameter refers to the whole molecule while the latter related to substituted groups. ¡ Chromatographic Parameters When the solubility of a solute is considerably greater in 1 st phase than the other , partition coefficient becomes difficult to determine experimentally. Chromatographic parameters obtained from RP thin layer chromatography are occassionaly used as substitutes for partition coefficient. ¡

Silica gel plate coated with hydrophobic phase is eluted with aqueous/organic solvent system of increasing water content. ¡ Rf values are converted to Rm values which are the true measure of lipophilicity from the following equation Rm = Log(1/Rf-1) ¡

The Rm values offer advantages compared to log P values. ¡ Used as substitute for partition coefficient in QSAR investigations. a)Compounds need not be pure. b) Only traces of materials needed c) A wide range of hydrophilic and hydrophobic congeners can be investigated d) The measurement of practicaly insoluble analogs possess no problem e) No quantitative method for concentration determination needed f) Several compounds can be estimated simultaneously ¡

¡ The main disadvantages are a) Lack of precision and reproducibility b) Use of different organic solvent system renders the derivation of π and f related scales impossible.

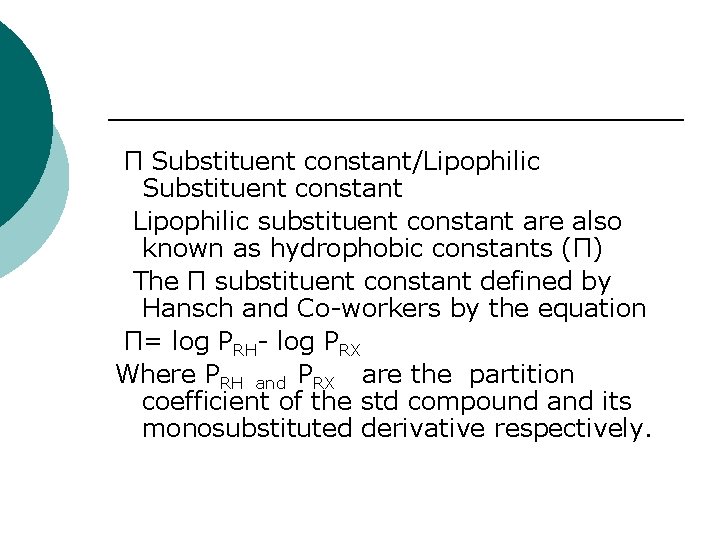
Π Substituent constant/Lipophilic Substituent constant Lipophilic substituent constant are also known as hydrophobic constants (Π) The Π substituent constant defined by Hansch and Co-workers by the equation Π= log PRH- log PRX Where PRH and PRX are the partition coefficient of the std compound and its monosubstituted derivative respectively.

Calculation of Log P value of mchlorotoluene is given as log P= log P benzene + π Cl + π me = 2. 13+0. 71+0. 56=3. 40 log P= log P toluene + π me- Cl = 2. 69+0. 76 =3. 45 log P= log P chlorobenzene + π meta- Me = 2. 84+0. 51=3. 35 log. P exp = 3. 28 ¡

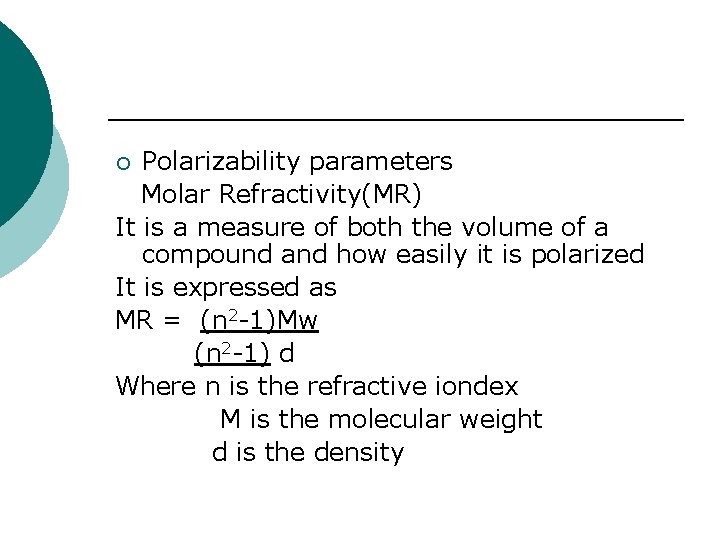
Polarizability parameters Molar Refractivity(MR) It is a measure of both the volume of a compound and how easily it is polarized It is expressed as MR = (n 2 -1)Mw (n 2 -1) d Where n is the refractive iondex M is the molecular weight d is the density ¡

The term Mw/d define a volume , while the term (n 2 -1) / (n 2+ 2) indicates a correction factor by indicating how easily the substituent can be polarised. ¡ This is particularly significant if the substituent has a π electron or lone pair of electrons. ¡

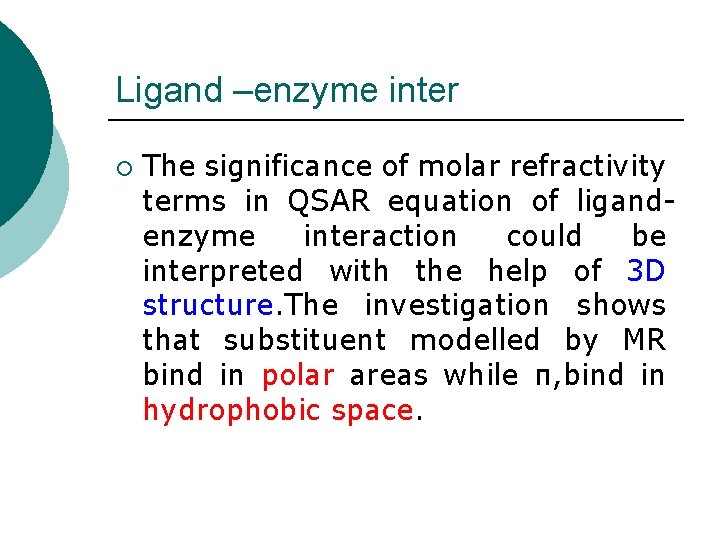
Ligand –enzyme inter ¡ The significance of molar refractivity terms in QSAR equation of ligandenzyme interaction could be interpreted with the help of 3 D structure. The investigation shows that substituent modelled by MR bind in polar areas while π, bind in hydrophobic space.

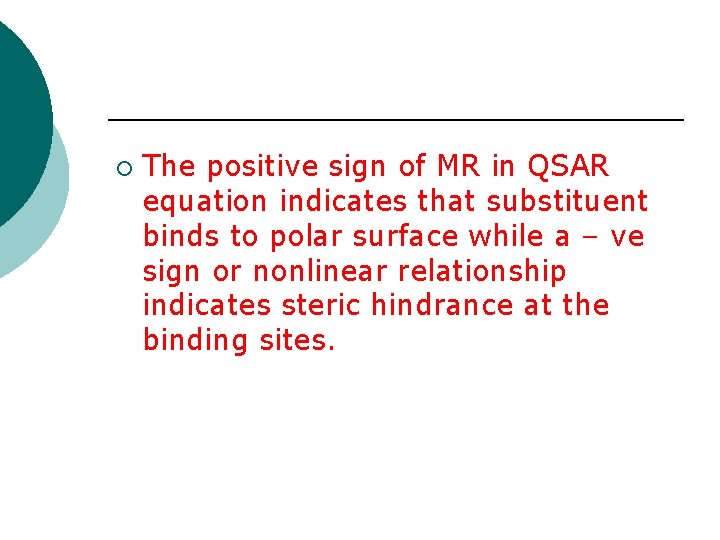
¡ The positive sign of MR in QSAR equation indicates that substituent binds to polar surface while a – ve sign or nonlinear relationship indicates steric hindrance at the binding sites.

¡ Electronic Parameters The distribution of electron in a drug molecule has considerable influence on the distribution and activity of the drug. Polar and nonpolar drug unionized form are transported through the membrane than polar drugs in their ionized form.

¡ ¡ If the drug reaches the target site, the distributed electron will control the type of bond that it forms with the target site, which inturn affects its biological activity. The Hammet Constant (σ) He quantified the electronic effects of groups on the physiochemical properties of the cpd. The distribution of the elecrons within a molecule depends on the nature of the electron withdrawing and donating group found in the structure.

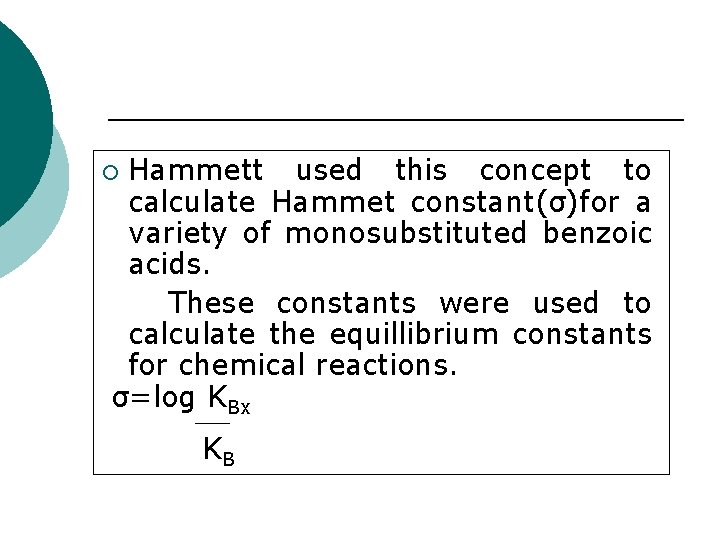
Hammett used this concept to calculate Hammet constant(σ)for a variety of monosubstituted benzoic acids. These constants were used to calculate the equillibrium constants for chemical reactions. σ=log KBx ¡ KB

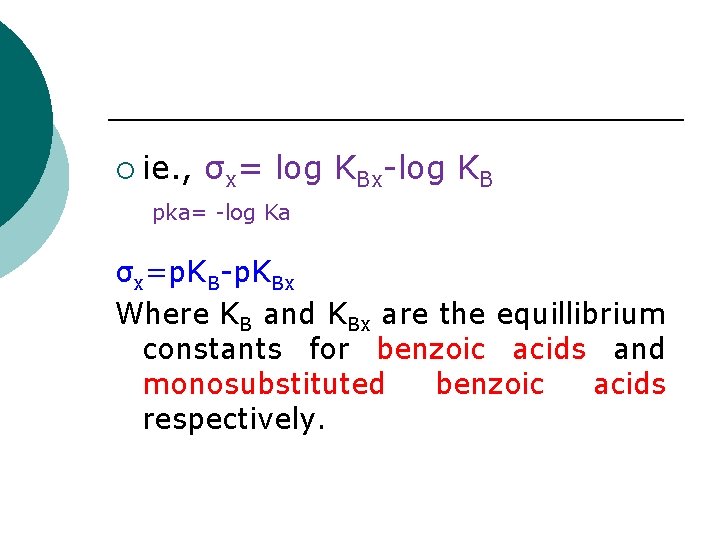
¡ ie. , σx= log KBx-log KB pka= -log Ka σx=p. KB-p. KBx Where KB and KBx are the equillibrium constants for benzoic acids and monosubstituted benzoic acids respectively.

¡ Hammett substituent const is a measure of the electron withdrawing or donating ability of a substituent and is determined by comparing the dissociation of a series of substituted acid with that of the parent or unsubstituted acid

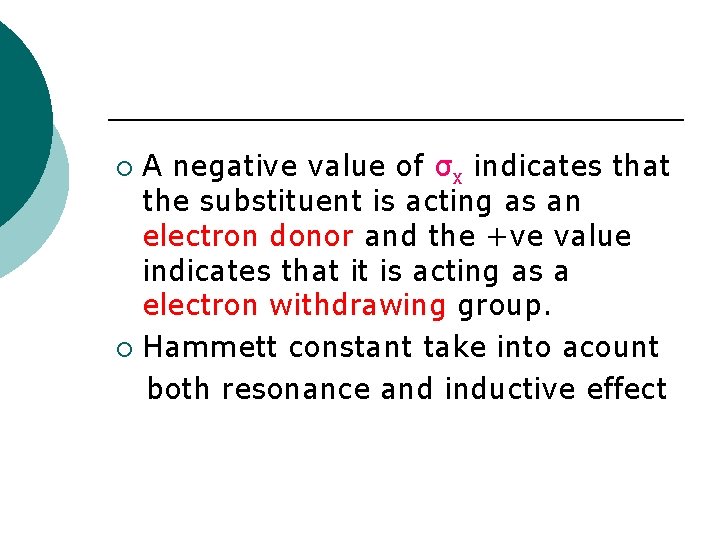
A negative value of σx indicates that the substituent is acting as an electron donor and the +ve value indicates that it is acting as a electron withdrawing group. ¡ Hammett constant take into acount both resonance and inductive effect ¡

¡ ¡ ¡ The values for σ for a particular substituent varies depending on their substitution meta or para. The meta and para sigma value are commonly used and indicated by a subscript m or p after the symbol , The o – substituent are unreliable due to steric hindrance and other effects such as intramolecular hydrogen bonding.

3 Kinds of static or electical influence states predominates ¡ Resonance or Mesomeric effect Inductive effect. ¡ Electrical effect which is transmitted by the polarization of electrons from atom to another. ¡

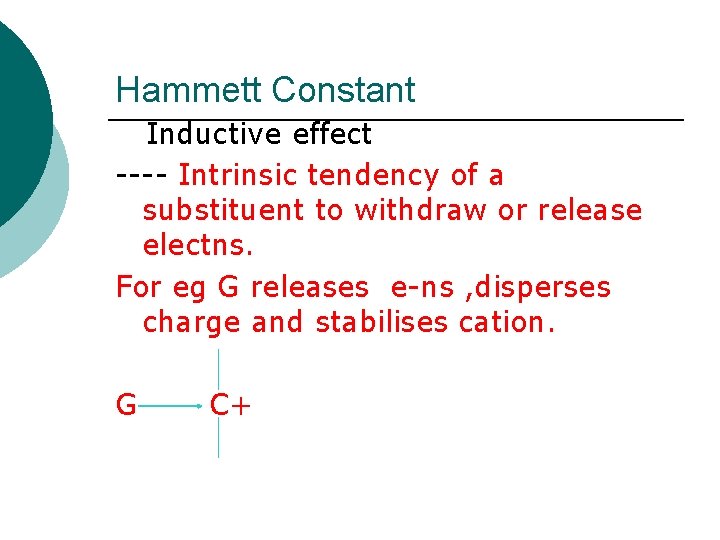
Hammett Constant Inductive effect ---- Intrinsic tendency of a substituent to withdraw or release electns. For eg G releases e-ns , disperses charge and stabilises cation. G C+

¡ G withdraws e-ns intensifies the charge and destabilizes the cation. G C+

Disadvantage: They only apply to substituent directly attached to benzene ring. If there is more than one substituent the value are summarized as Σσ. This constant was unsuccessful to relate biological activity sine the electron distribution is not the only factor involved.

Inductive substituent Constant: Hammett constant are a measure of both inductive and mesomeric effect. The para substituent constant (σp) has a greater resonance component than the equivalent meta constant (σm) and the inductive const can be calculated from the inductive substituent constant (σi) ¡ σi= ½ (3 σp- σm)

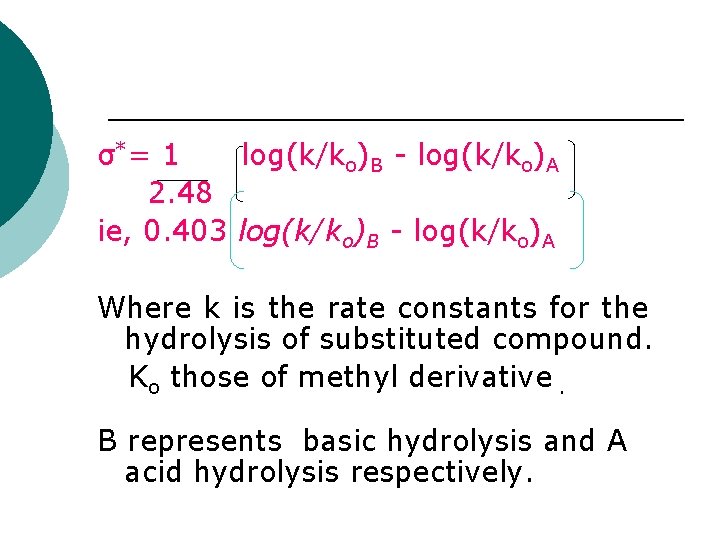
It is used in aliphatic compound where influencing and influenced form group do not form a part of the conjugated system. Taft’s substituent constant( σ*) It is a measure of the polar effects of a substituent in aliphatic compound. They are based on the hydrolysis of ester and calculated frm the following eqn ¡

σ* = 1 log(k/ko)B - log(k/ko)A 2. 48 ie, 0. 403 log(k/ko)B - log(k/ko)A Where k is the rate constants for the hydrolysis of substituted compound. Ko those of methyl derivative. B represents basic hydrolysis and A acid hydrolysis respectively.

The factor 1/2. 48 is a constant B is influenced by the polar effect so that by substracting the acid term from the basic term only the polar effect remain. In taft’s substituent constant only the methyl group is the standard for which the constant is zero. ¡

¡ That can be compared with other constant by writing the methyl group in the form CH 2 -H and identify it as the group for H. Taft and Inductive substituent constant are related as σ*= 2. 51 σi

¡ Steric Substitution Constant The drug approaches the binding site to interact with the receptor. The bulk, size and shape of the drug may influence on this process. A steric substitution constant is a measure of the bulkiness of the grp it represents and its effect on the closeness of the group between the drug and receptor site.

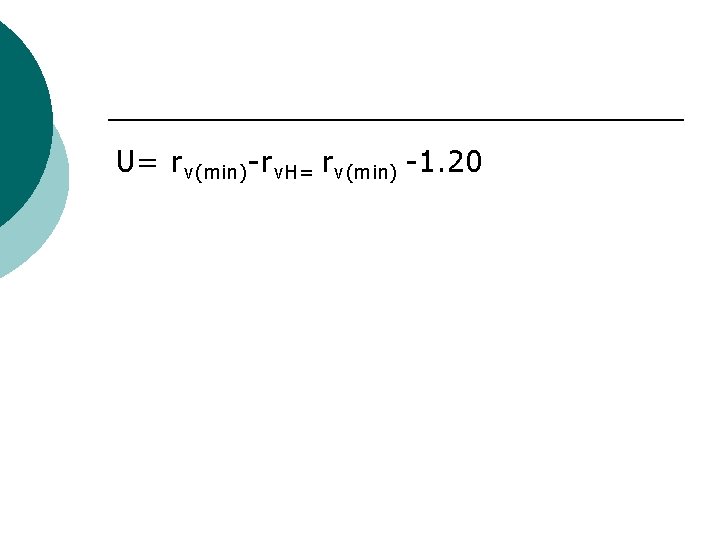
CHARTON’S STERIC CONSTANT ¡ Corrected Vanderwaal’s radius U in which the minimum Vanderwaal’s radii of the substituent grp (rv(min) is corrected for the corresponding radius for hydrogen(rv. H),

U= rv(min)-rv. H= rv(min) -1. 20

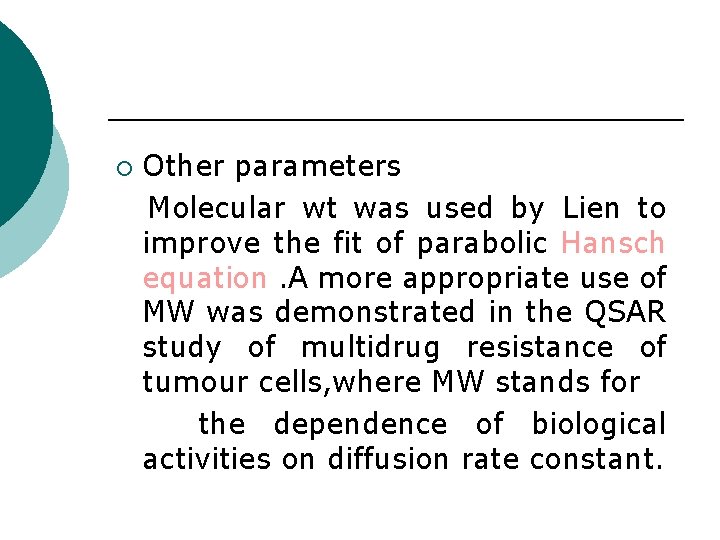
¡ Other parameters Molecular wt was used by Lien to improve the fit of parabolic Hansch equation. A more appropriate use of MW was demonstrated in the QSAR study of multidrug resistance of tumour cells, where MW stands for the dependence of biological activities on diffusion rate constant.

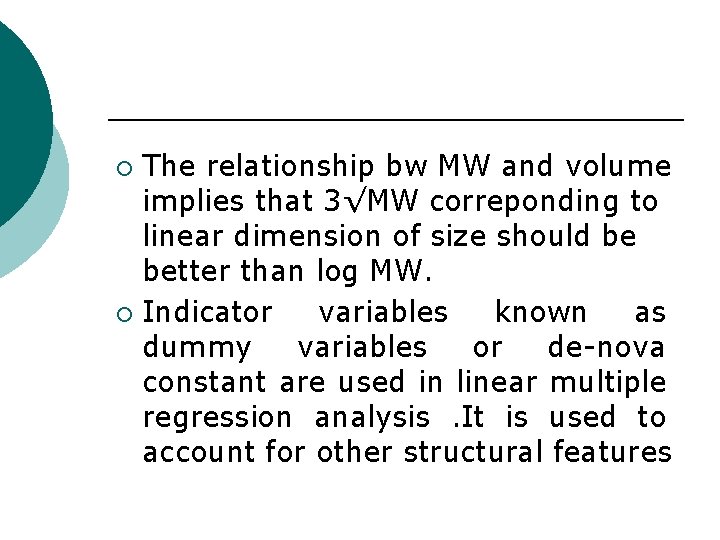
The relationship bw MW and volume implies that 3√MW correponding to linear dimension of size should be better than log MW. ¡ Indicator variables known as dummy variables or de-nova constant are used in linear multiple regression analysis. It is used to account for other structural features ¡

1. Intramolecular hydrogen bonding, hydrogen donor & acceptor properties, ortho effects, cis/trans isomers, diff parent skeleton, diff test models etc.

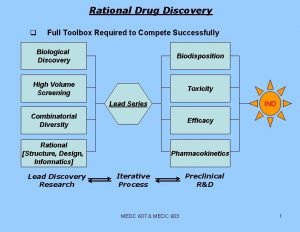
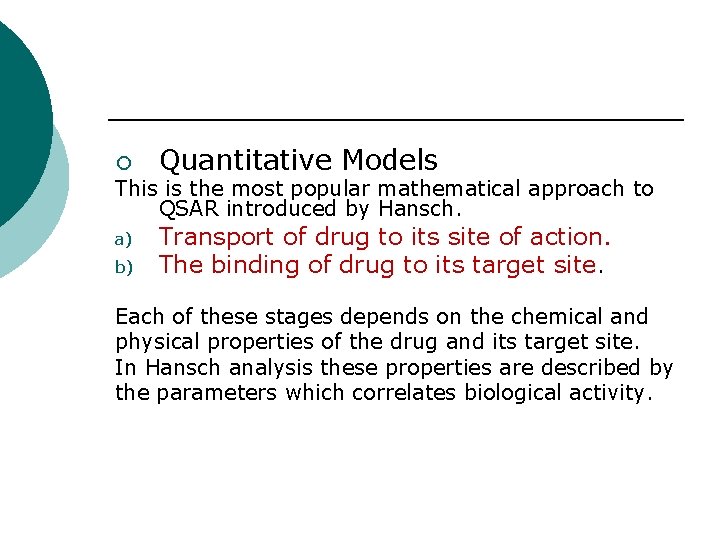
¡ Quantitative Models This is the most popular mathematical approach to QSAR introduced by Hansch. a) b) Transport of drug to its site of action. The binding of drug to its target site. Each of these stages depends on the chemical and physical properties of the drug and its target site. In Hansch analysis these properties are described by the parameters which correlates biological activity.

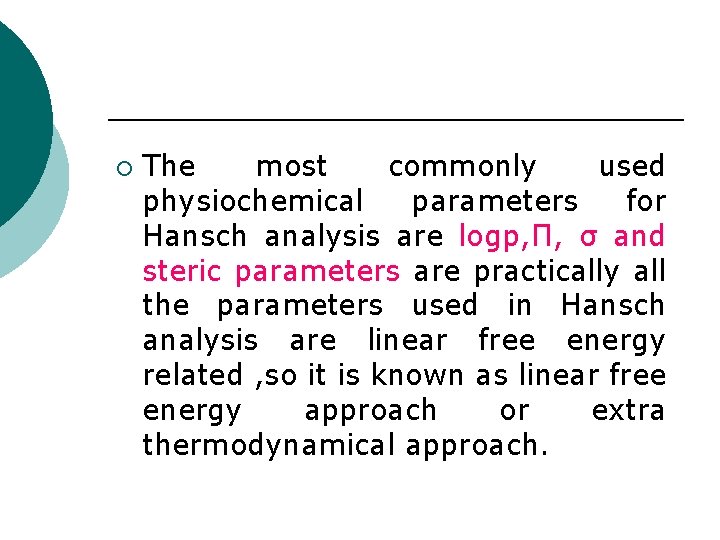
¡ The most commonly used physiochemical parameters for Hansch analysis are logp, Π, σ and steric parameters are practically all the parameters used in Hansch analysis are linear free energy related , so it is known as linear free energy approach or extra thermodynamical approach.

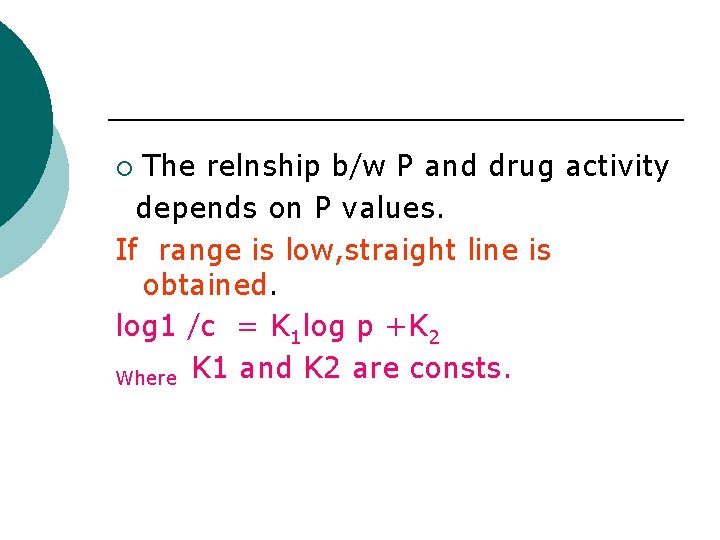
The relnship b/w P and drug activity depends on P values. If range is low, straight line is obtained. log 1 /c = K 1 log p +K 2 Where K 1 and K 2 are consts. ¡

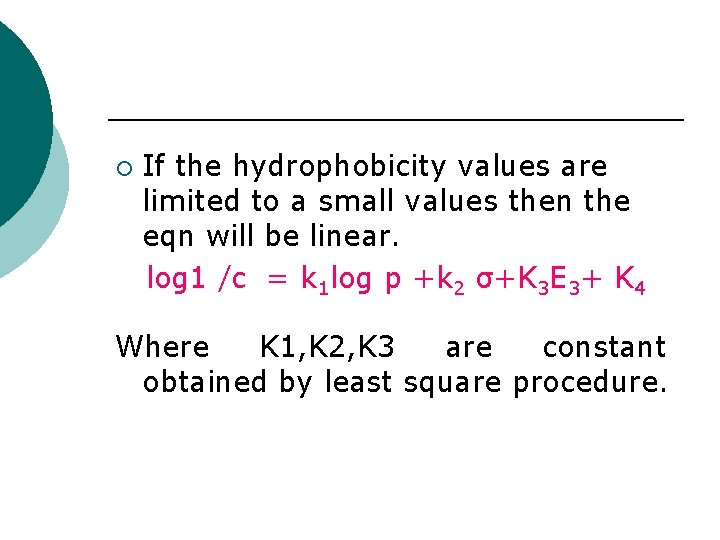
¡ If the hydrophobicity values are limited to a small values then the eqn will be linear. log 1 /c = k 1 log p +k 2 σ+K 3 E 3+ K 4 Where K 1, K 2, K 3 are constant obtained by least square procedure.

C is the molar concentration that produces biological action. The molecule which are too hydrophilic or lipophilic will not be able to cross the lipophilic or hydrophilic barriers respectively. If the P value are higher range , then the equation will be parabolic and given as ¡

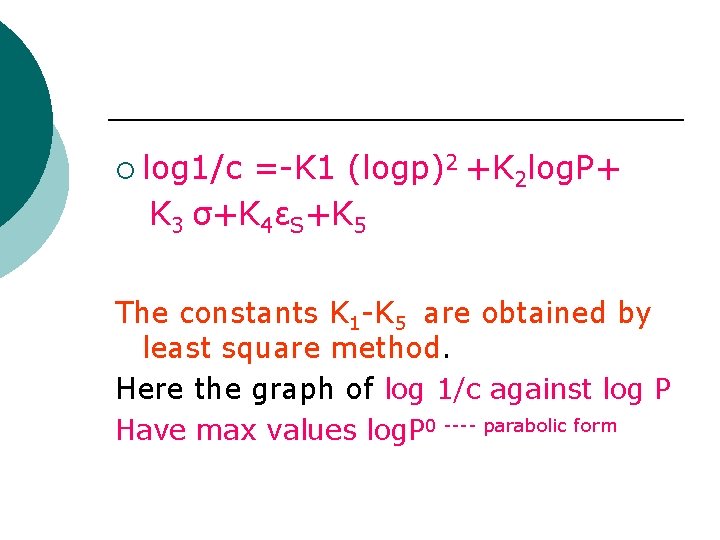
¡ log 1/c =-K 1 (logp)2 +K 2 log. P+ K 3 σ+K 4εS+K 5 The constants K 1 -K 5 are obtained by least square method. Here the graph of log 1/c against log P Have max values log. P 0 ---- parabolic form

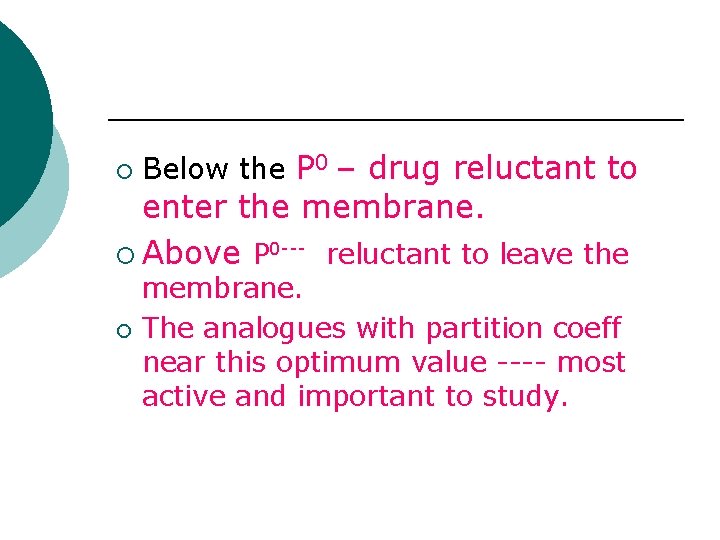
¡ Below the P 0 – drug reluctant to enter the membrane. ¡ Above P 0 --- reluctant to leave the ¡ membrane. The analogues with partition coeff near this optimum value ---- most active and important to study.

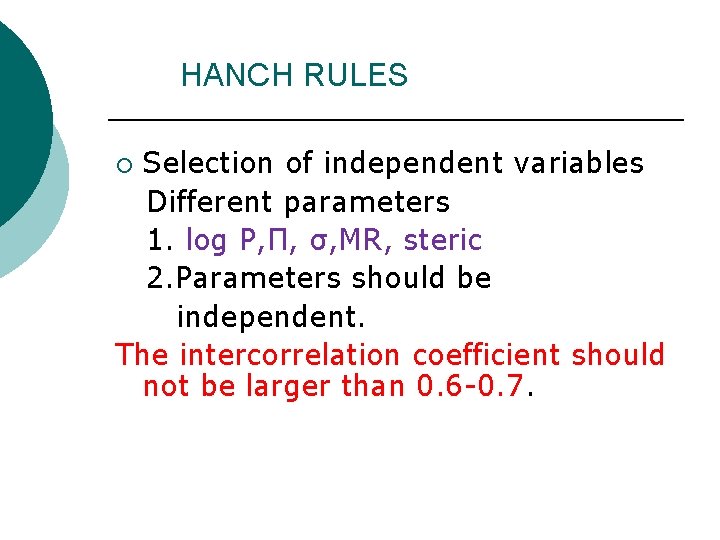
HANCH RULES Selection of independent variables Different parameters 1. log P, Π, σ, MR, steric 2. Parameters should be independent. The intercorrelation coefficient should not be larger than 0. 6 -0. 7. ¡

¡ All the reasonable parameters must be validated by appropriate statistical procedure ie either by a step wise regression analysis or cross validation.

¡ The best equation is normally one with lower standard deviation and higher F value.

¡ If all the equations are equal simplest one is taken ¡ Number of variable should be at least 5 or 6 data point per variable to avoid chance correlations. ¡ Model should be consistent with the known physical. organic and bio-medicinal chemistry of the process under consideration.

¡ APPLICATIONS OF HANCH ANALYSIS It is used to predict the activity of an yet unsynthesised analogue. This enables the medicinal chemist to synthesise an analogue which is worthy.

Eg; The adrenergic activity of a series of analogues of βhaloarylamine was observed. It was found that only pi and sigma values only related to activity and not the steric factor. Log 1/c =1. 78 pi+1. 674 ¡

¡ Hansch analysis may also used to give an indication of the importance of the influence of parameters on the mechanism by which a drug acts.

The smaller the value of coefficient of σ relative to that of Π in tha above eqn shows that electronic effect do not play an important role in the action of the drug. The accuracy of Hansch equation depends on 1)The no of analogues used ¡

2) The greater the number, the higher the probability of obtaining an accurate Hansch equation. 3)The accuracy of biological data used in the derivation of the equation. 4)The choice of parameters.

¡ Free Wilson Analysis It is a true SAR model. This is an alternative approach to Hamett model. Free and Wilson used a series of substituent constant which related biological activity to the presence of a specific functional group at a specific location on the parent molecule.

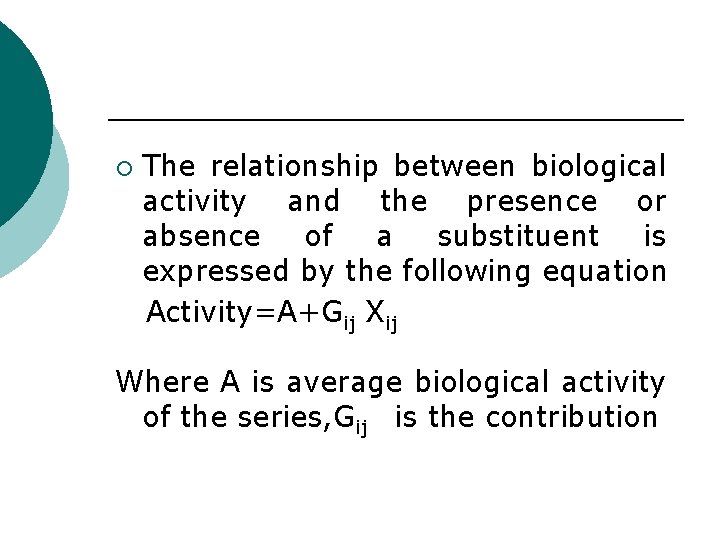
¡ The relationship between biological activity and the presence or absence of a substituent is expressed by the following equation Activity=A+Gij Xij Where A is average biological activity of the series, Gij is the contribution

of actiivity to the functional grp i in the jth position and Xij the presence or absence of functional grp i in the jth position. Whole molecular descriptors Some of the natural

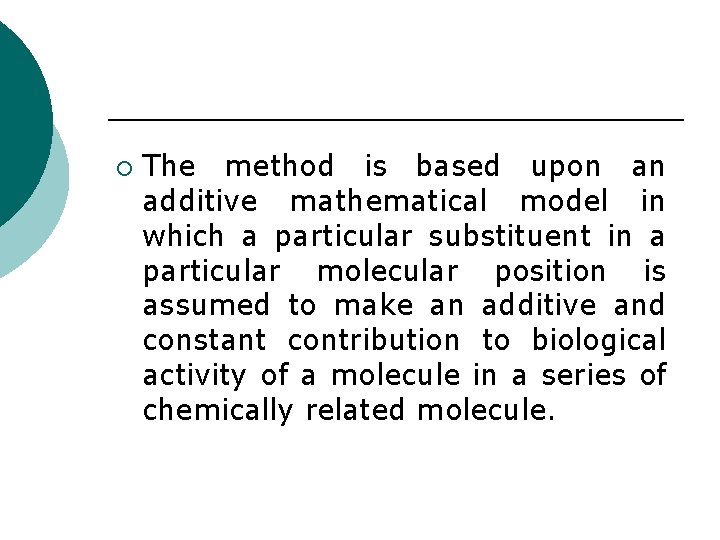
¡ The method is based upon an additive mathematical model in which a particular substituent in a particular molecular position is assumed to make an additive and constant contribution to biological activity of a molecule in a series of chemically related molecule.

Mathematical model, Additive model or denovo approach are the synonyms for free wilsonmethod. The substituent constant based on activities are used rather than physical properties

¡ Introduction of a particular substituent at a particular molecular position always lead to a quantitatively similar effect on biological potency of the whole molecules and expressed by the eqn as BA=µ+Σaij

¡ Where µ= contribution of unsubstituted compounds Σaij = contribution of substituted compounds i = number of position the substituent occurs j = number of substituent at that position

APPLICATION 1)Easy to apply 2)Simple method to derive the substituent contribution and to have a first look on their possible dependence on different physiochemical properties

The substituent which cannot fulfill the principle of additivity can be recognized. ¡ Substituent constants like σ and Π were not considered and so this method is effective when substituent constants are not available. ¡

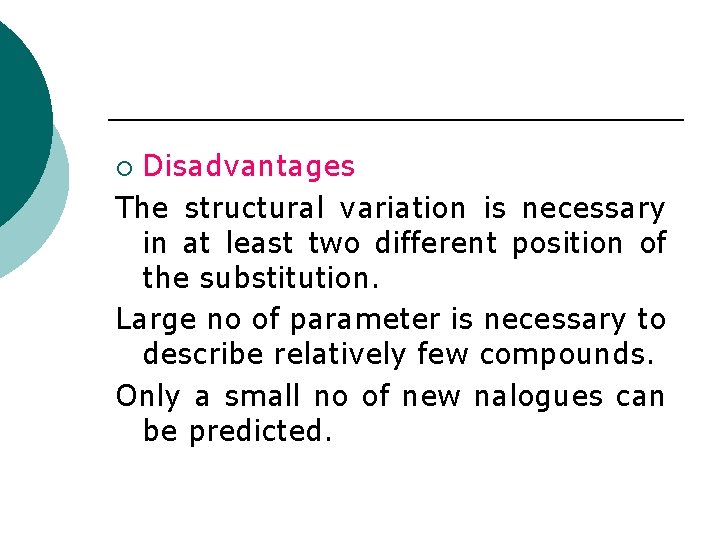
Disadvantages The structural variation is necessary in at least two different position of the substitution. Large no of parameter is necessary to describe relatively few compounds. Only a small no of new nalogues can be predicted. ¡
 Hansch analysis in qsar
Hansch analysis in qsar Drug discovery process
Drug discovery process Topliss scheme
Topliss scheme Selexyz dominicanen maastricht
Selexyz dominicanen maastricht Difference between qsar and sar
Difference between qsar and sar Accidental adulteration definition
Accidental adulteration definition Qsar
Qsar Log 1/c qsar
Log 1/c qsar Oecd qsar toolbox
Oecd qsar toolbox Qsar
Qsar Qsar toolbox 매뉴얼
Qsar toolbox 매뉴얼 Novel clinical drug trial design
Novel clinical drug trial design Cadd drug design
Cadd drug design Biopharmaceutic considerations in drug product design
Biopharmaceutic considerations in drug product design Computer aided drug design lecture notes
Computer aided drug design lecture notes Biodisposition definition
Biodisposition definition Life is a highway metaphor meaning
Life is a highway metaphor meaning The greatest contributors to our present gymnastics program
The greatest contributors to our present gymnastics program System and form design example
System and form design example Developed country
Developed country Water jet velocity formula
Water jet velocity formula Unix was originally developed in
Unix was originally developed in Comparative education in developed countries
Comparative education in developed countries Social bonding theory
Social bonding theory The circuit training method was developed in
The circuit training method was developed in What was the first tool of aeronautics to be developed?
What was the first tool of aeronautics to be developed? Wagner chronic care model
Wagner chronic care model Developed country
Developed country Unconditioned stimulus unconditioned response
Unconditioned stimulus unconditioned response Mdc and ldc
Mdc and ldc Cocomo i
Cocomo i Bulk temperature
Bulk temperature Html developed by
Html developed by Fundamentals of fluid mechanics
Fundamentals of fluid mechanics The expectancy model is developed by -
The expectancy model is developed by - Least developed country
Least developed country Which was the most important industry in the sangam age
Which was the most important industry in the sangam age Idealized self example
Idealized self example Designed & developed by gstn
Designed & developed by gstn Who invented control charts
Who invented control charts Eaf in cocomo model
Eaf in cocomo model The internal loading can be found by
The internal loading can be found by End user computing audit program
End user computing audit program Difference between ansi c and k&r c
Difference between ansi c and k&r c The basic structure of computer was developed by
The basic structure of computer was developed by Joseph luft and harry ingham in 1955
Joseph luft and harry ingham in 1955 Self actualized celebrities
Self actualized celebrities Mrtslk
Mrtslk Balanced scorecard of starbucks
Balanced scorecard of starbucks Gui is an interface between
Gui is an interface between Anne boykin theory
Anne boykin theory Abraham maslow
Abraham maslow State laws of dry friction
State laws of dry friction Incas developed ability
Incas developed ability Abraham maslow developed his hierarchy of needs
Abraham maslow developed his hierarchy of needs What is the theme of the fox and the goat
What is the theme of the fox and the goat William d pattison
William d pattison 1917 ffa history
1917 ffa history Designed & developed by gstn
Designed & developed by gstn Bar chart advantages and disadvantages
Bar chart advantages and disadvantages Quantum mechanical model scientist
Quantum mechanical model scientist This is the basic motion?
This is the basic motion? It is the alignment of the ideal and real self.
It is the alignment of the ideal and real self. Why was the gps network originally developed
Why was the gps network originally developed Ccf was developed by unesco
Ccf was developed by unesco The arbitrage pricing theory was developed by
The arbitrage pricing theory was developed by A carefully developed overall approach to leading
A carefully developed overall approach to leading Who developed the assure model
Who developed the assure model Origin of number system
Origin of number system Well developed paragraph format
Well developed paragraph format Which chef created the brigade system
Which chef created the brigade system What is the climax of the gift of the magi
What is the climax of the gift of the magi Less developed countries
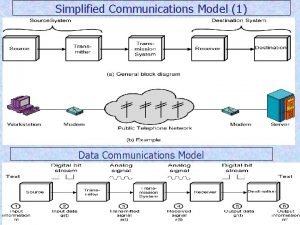
Less developed countries Explain simplified data communication model
Explain simplified data communication model Sunrise model transcultural nursing
Sunrise model transcultural nursing Multi floor plant layout evaluation was developed
Multi floor plant layout evaluation was developed Less developed countries
Less developed countries Piche evaporimeter diagram
Piche evaporimeter diagram Alchemists developed processes for separating
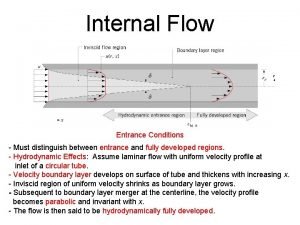
Alchemists developed processes for separating Internal flow examples
Internal flow examples