QP Forum Regulatory Update Paul Sexton GMP Manager





















- Slides: 26

QP Forum Regulatory Update Paul Sexton, GMP Manager, HPRA Trinity College Dublin – 12 th April 2018

Content • Revised Statement of Non Compliance • Control of Cross Contamination - Q&A • Safety Features • Annex 21 – Importation • MRA - EU / US • Brexit Update

Statement of Non Compliance (SNC) Revised SNC issued for consultation (3 rd April – 15 th May) - QRM to facilitate continued supply of critical medicines - Risk / Benefit report on use of product with involvement of: Marketing Authorisation Holder Manufacturing / Importer Authorisation Holder (Site of batch certification) QP, Manufacturer which is subject of SNC (if different) - Report sent to relevant National Competent Authorities (NCA) in affected markets. - Written confirmation of product criticality and no objection to distribution from National Competent Authority in market concerned. - Supervisory Authority informed.

Control of Cross Contamination - Q&A on implementation of risk based prevention of cross contamination and use of the Safety Working Party guidance on use of Health Based Exposure Limits. http: //www. ema. europa. eu/docs/en_GB/document_library/Other/2017/01/WC 500219500. pdf (original Q&A) • Consultation on Q&As : January – April 2017 • Meeting with industry representatives : June 20 & 21 st, 2017 Discussion Points - classification of ‘highly hazardous’ products - use of 1/1000 th minimum therapeutic dose

Control of Cross Contamination - Q&A • Q&As revised based on feedback • Undergoing final approval process at EMA • Anticipate publication on EMA website in Quarter 2, 2018

Safety Features Two elements 1. Unique identifier for individual pack (2 D barcode) 2. Anti Tampering Device Rules applicable from 9 th Feb 2019 Commission Publication Safety Features for Medicinal Products for Human Use Questions & Answers (Version 9) https: //ec. europa. eu/health/sites/health/files/falsified_medicines/qa_safetyfeature_v 9. pdf 28/11/2020 6

GMP Guidance Updates Annex 21 – Importation of Medicinal Products - Outsourced activity - Oversight of manufacturers - Technical / Quality agreements - Site of batch certification (MIA required) - Site of physical importation (MIA required) - Sampling of imported products (Annex 16)

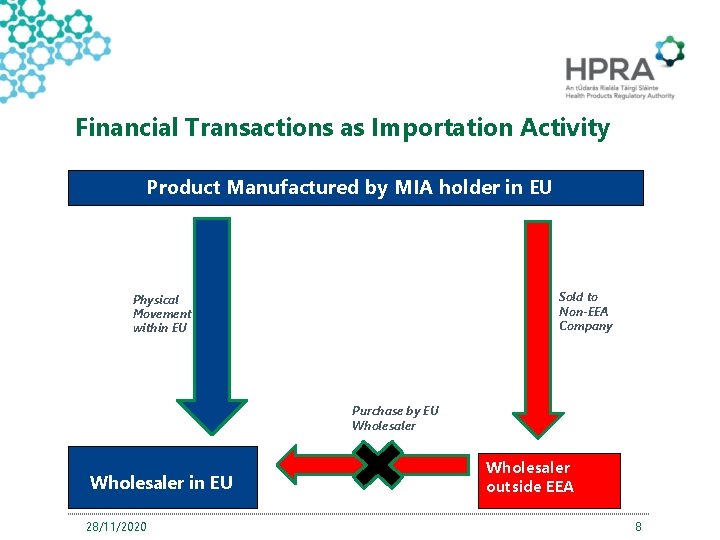
Financial Transactions as Importation Activity Product Manufactured by MIA holder in EU Sold to Non-EEA Company Physical Movement within EU Purchase by EU Wholesaler in EU 28/11/2020 Wholesaler outside EEA 8

Financial Transactions as Importation Activity Purchase of product from entity in 3 rd Country • Discussed previously at GMDP IWG • Some Member States have implemented MIA requirement • HPRA has not issued MIAs yet for this activity - New area for consideration 28/11/2020 9

Financial Transactions as Importation Activity Commission Q&A on Safety Features (Version 9 ) • 5. 5. Question: Article 22(a) requires a wholesaler to verify the authenticity of and decommission the unique identifier of all medicinal products he intends to distribute outside of the Union. Is it necessary to decommission the unique identifier if the medicinal product is sold to a party established outside the EU but that product does not physically leave the wholesaler's premises in the EU? • Answer: No. The purpose of Article 22(a) is to ensure the decommissioning of unique identifiers on packs which leave the EU territory, in order to avoid that those active codes may be harvested by traffickers. In case the medicinal product is sold to a party established outside the EU but physically remains in the wholesaler's premises in the EU, the unique identifier on the product should not be decommissioned. If that medicinal product is subsequently imported (while physically remaining in the EU) by a holder of a manufacturing and import authorisation (a wholesaler cannot import medicinal products), no action is required with regard to the safety features. 28/11/2020 10

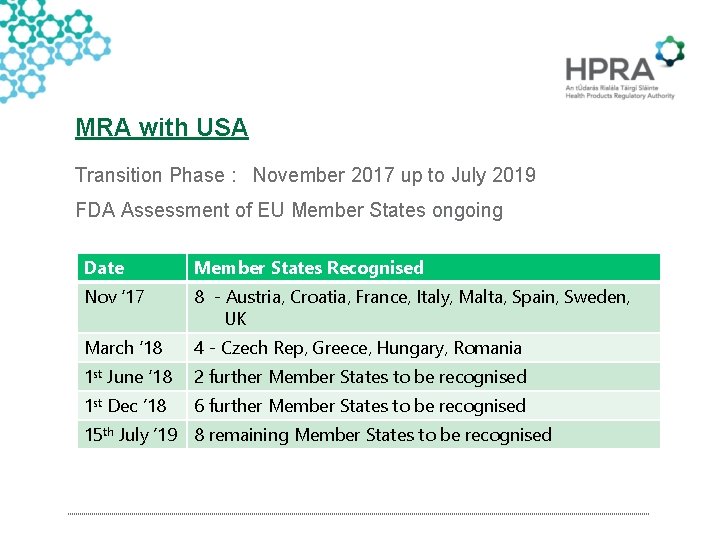
MRA with USA Transition Phase : November 2017 up to July 2019 FDA Assessment of EU Member States ongoing Date Member States Recognised Nov ‘ 17 8 - Austria, Croatia, France, Italy, Malta, Spain, Sweden, UK March ‘ 18 4 - Czech Rep, Greece, Hungary, Romania 1 st June ‘ 18 2 further Member States to be recognised 1 st Dec ’ 18 6 further Member States to be recognised 15 th July ’ 19 8 remaining Member States to be recognised

EU – US MRA Inspection Activity No routine inspections by EU Authorities in US for products within scope of the MRA HPRA will continue to conduct inspections for products outside scope of the MRA excludes – vaccines, blood products, veterinary products (to be considered for inclusion within specified timeframes) Voluntary recognition of inspections in Third Countries possible under the MRA. Sources of information - EMA website Q&A on the MRA http: //www. ema. europa. eu/docs/en_GB/document_library/Other/2017/10/WC 500237908. pdf 28/11/2020 12

EU – US MRA Marketing Authorisation Applications / Variations No GMP certificates issued by the FDA for US sites. Certificate of Pharmaceutical Product (CPP) - Also referred to as Export Certificates - WHO format document & valid for 2 years from issue date. - Should be clear that the scope of the CPP includes the US based manufacturing site. EMA Q&A on impact of EU-US MRA on MA applications & variations http: //www. ema. europa. eu/docs/en_GB/document_library/Other/2017/07/WC 500232411. pdf 28/11/2020 13

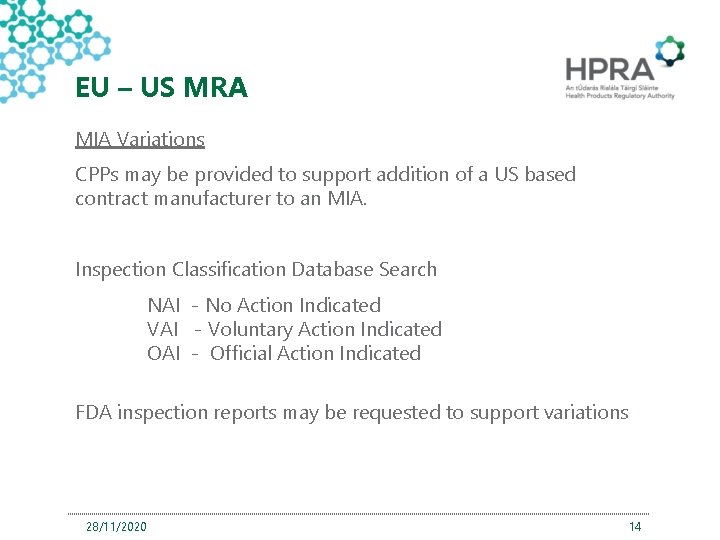
EU – US MRA MIA Variations CPPs may be provided to support addition of a US based contract manufacturer to an MIA. Inspection Classification Database Search NAI - No Action Indicated VAI - Voluntary Action Indicated OAI - Official Action Indicated FDA inspection reports may be requested to support variations 28/11/2020 14

Brexit Regulatory Update • Supply Aspects • Actions at EU Level • Impact for EMA • HPRA Priorities • Transfer of EU batch release sites to IE • FAQs regarding QPs • MAH transfers • Points for consideration

Supply Aspects Products Transiting Through the UK • Ireland has a unique geographic location with UK land border and reliance of the UK landbridge • Transportation of medicines by road through the UK Ireland ↔ Mainland Europe • Estimates of 80% of trade to and from Ireland in 2016 via UK • Additionally, 60% (by value) of airfreight with a 3 rd country destination was exported via the UK. • 44% of all imports (air and sea) from 3 rd countries arrived via the UK. • Import status of product transiting through the UK ? ?

Supply Aspects • Ireland – small market • Common language with UK • Significant number of products have dual pack labelling (Irish & UK markets) • Some MAs may not be maintained for the Irish market for commercial reasons 28/11/2020 17

Supply Aspects • Currently wholesale trade exists between Ireland UK – Common Labelling – e. g. Dual Pack Labelling or CAPs – Free movement of batches without additional QP certification • Wholesalers are not authorised to import from 3 rd Countries • Potential for delays in supply and product shortages

Actions at EU Level • IWG subgroup established • Looking at impact for EU 27 and UK • Focus is to avert potential product shortages • Competent Authorities (CA) need adequate resourcing to deal with additional workload created by Brexit.

Impact of Brexit for EMA • Relocation of the EMA to Amsterdam • Relocation of EMA staff • Reduction in GMDP IWG meetings (3 for 2018) • Similar reduction in meetings across other areas

HPRA Priorities • Patients & animals - committed to ensuring continuity of supply of medicines • Engagement with industry, associations and Government departments to facilitate trade • Participation in EU Brexit working groups to collaborate on guidance, Q&As etc. • Work with individual companies, as requested, to discuss their plans for changes 28/11/2020 21

Transfer of EU ‘batch release’ site to IE • A number of companies are relocating the responsibility for EU batch certification of products from the UK to Ireland • New applications should be submitted 6 months before the Brexit date or the end of transition period, as appropriate. • The HPRA also encourages intending applicants to make early contact with the Compliance Department by emailing inspect@hpra. ie prior to submission of the application 28/11/2020 22

FAQs regarding QP • Does the QP need to be an Irish resident? – No, the EU directives don’t include this requirement and the HPRA does not have a national policy setting a requirement for residency • Can the QP work from another jurisdiction? – The batch certification should take place at the premises detailed on the MIA as this is a prescribed activity and under Irish law must take place at that address. Batch certification cannot be delegated to anyone who is not a named QP on the MIA • Will a QP, who is named on a UK licence, be accepted here? – The educational qualifications would be accepted if they had been named on a UK MIA. Separate assessment would be undertaken of their experience in relation to the dosage forms to be certified. • Does a company need to obtain an MIA in IE to perform batch certification? – No , they may contract another MIA holder to perform the certification on their behalf. The site at which certification takes place would be named on the PIL. 28/11/2020 23

MAH Transfers • Zero day’ policy will not apply to MAH transfers resulting from Brexit – MAHs be allowed up to 6 months to implement packaging changes following issue of the transferred application – Reference to rundown and recall of product bearing the ‘old’ MA number will be removed from the transfer forms and guidance documents 28/11/2020 24

Points for consideration • HPRA recommend that QPs consider how to manage this transition at site level • If Irish site takes on additional products the QP may also request data to support their role e. g. previously reported quality defects, PQRs, etc. • Contractual arrangements may be required to address on-ward reporting of complaints and ADRs, if required 28/11/2020 25

Thanks for your attention Questions ? ? ?
 Paul sexton wholesale cars
Paul sexton wholesale cars Is an alternative of log based recovery
Is an alternative of log based recovery Rhonda sexton
Rhonda sexton Aerosol overcaps
Aerosol overcaps Sexton engineering
Sexton engineering Whitney sexton
Whitney sexton Jill sexton
Jill sexton Dan sexton civil service
Dan sexton civil service Dr sexton vancouver
Dr sexton vancouver Cro manager forum
Cro manager forum Portfolio manager synergy manager parental developer
Portfolio manager synergy manager parental developer Senior manager vs general manager
Senior manager vs general manager Paul smith football manager
Paul smith football manager Dobra praktyka wytwarzania
Dobra praktyka wytwarzania Principles of gmp
Principles of gmp Gmp for active pharmaceutical ingredients
Gmp for active pharmaceutical ingredients Gmp stands for
Gmp stands for Gmp
Gmp What are the 10 principles of gmp?
What are the 10 principles of gmp? Gmp sterile pharmaceutical products
Gmp sterile pharmaceutical products Gmp class d
Gmp class d Components of gmp
Components of gmp Gmp sterile pharmaceutical products
Gmp sterile pharmaceutical products Objectives of gmp
Objectives of gmp Gmp in pharma
Gmp in pharma Chamilo gmp
Chamilo gmp Gmp means
Gmp means