QM of Particles III Rydberg atom With laser

QM of Particles III Rydberg atom With laser beams • News, Quiz • More on the Bohr model • Clicker questions on the Bohr model and energy quantization. 1

For LIGO for the direct observation of gravity waves Rainer Weiss MIT Barry Barish Caltech Kip Thorne Caltech
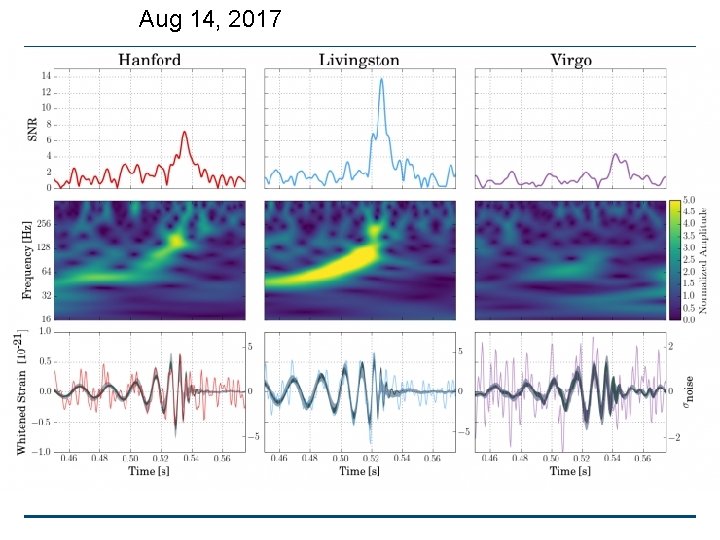
Aug 14, 2017

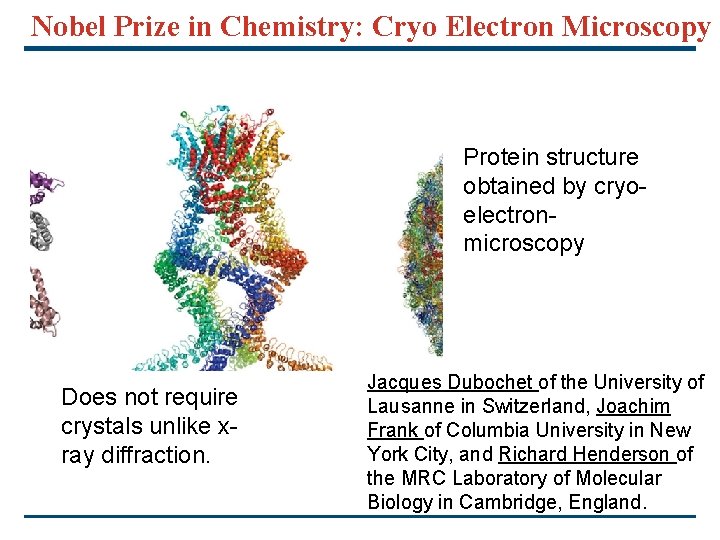
Nobel Prize in Chemistry: Cryo Electron Microscopy Protein structure obtained by cryoelectronmicroscopy Does not require crystals unlike xray diffraction. Jacques Dubochet of the University of Lausanne in Switzerland, Joachim Frank of Columbia University in New York City, and Richard Henderson of the MRC Laboratory of Molecular Biology in Cambridge, England.

Q 20. 1 These are the energy levels of a hypothetical atom. Initially the atom is in its ground state. If the atom absorbs a 18 e. V photon, what is the final state of the atom? A. B. C. D. n=2 n=3 n=4 None of the above 6

Q 20. 1 These are the energy levels of a hypothetical atom. Initially the atom is in its ground state. If the atom absorbs a 18 e. V photon, what is the final state of the atom? A. B. C. D. n=2 n=3 n=4 None of the above 7
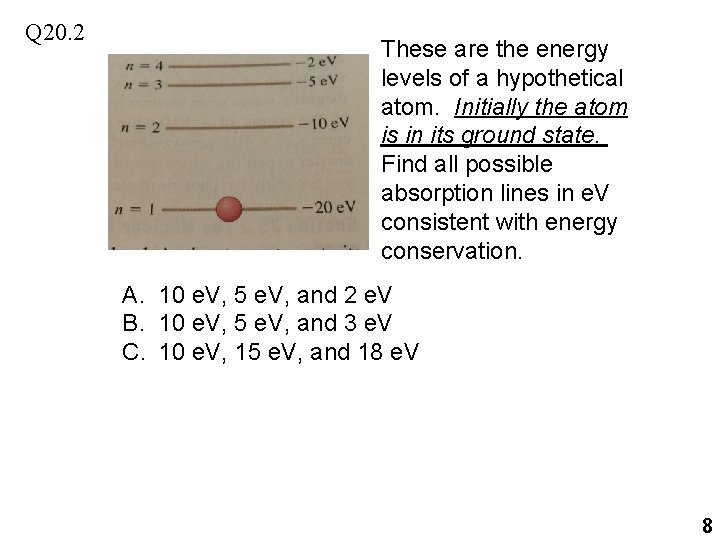
Q 20. 2 These are the energy levels of a hypothetical atom. Initially the atom is in its ground state. Find all possible absorption lines in e. V consistent with energy conservation. A. 10 e. V, 5 e. V, and 2 e. V B. 10 e. V, 5 e. V, and 3 e. V C. 10 e. V, 15 e. V, and 18 e. V 8
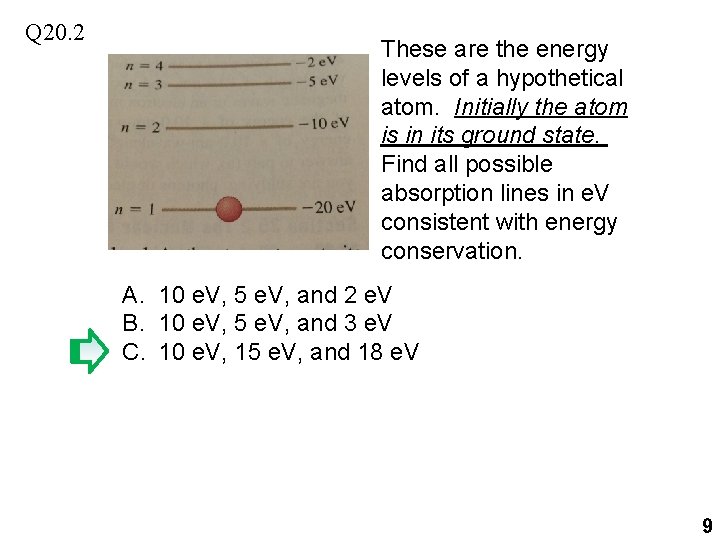
Q 20. 2 These are the energy levels of a hypothetical atom. Initially the atom is in its ground state. Find all possible absorption lines in e. V consistent with energy conservation. A. 10 e. V, 5 e. V, and 2 e. V B. 10 e. V, 5 e. V, and 3 e. V C. 10 e. V, 15 e. V, and 18 e. V 9
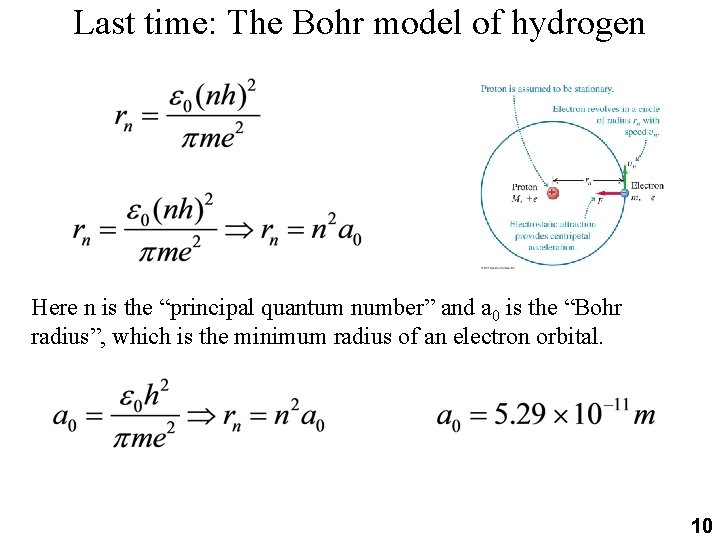
Last time: The Bohr model of hydrogen Here n is the “principal quantum number” and a 0 is the “Bohr radius”, which is the minimum radius of an electron orbital. 10
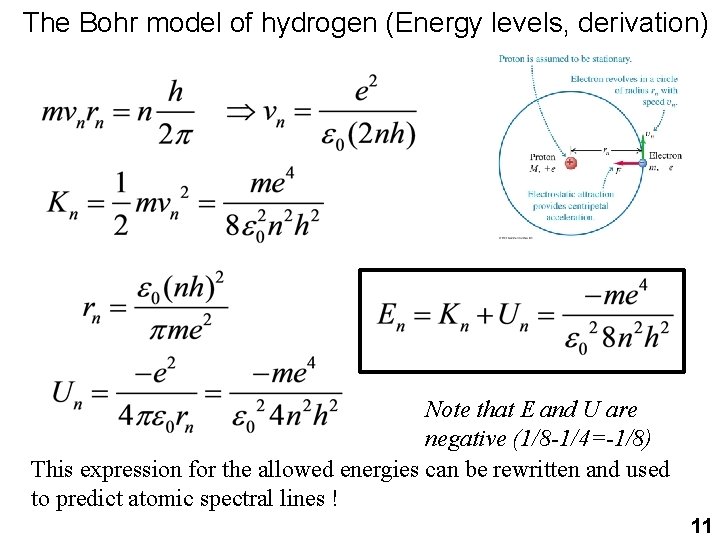
The Bohr model of hydrogen (Energy levels, derivation) Note that E and U are negative (1/8 -1/4=-1/8) This expression for the allowed energies can be rewritten and used to predict atomic spectral lines ! 11
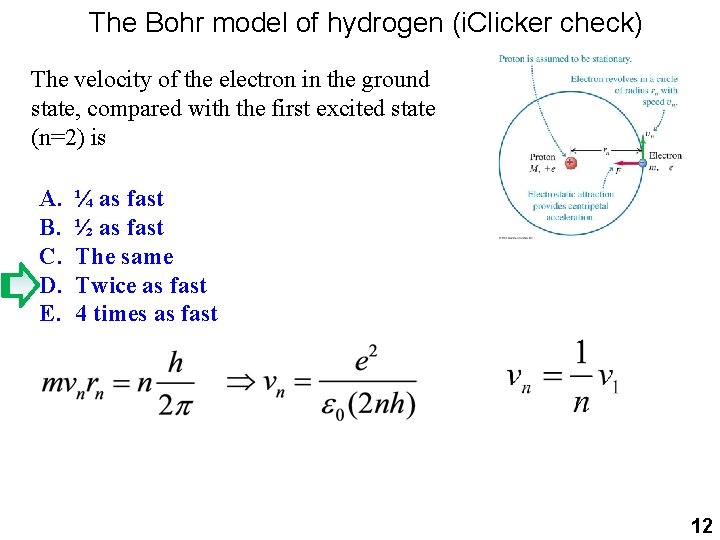
The Bohr model of hydrogen (i. Clicker check) The velocity of the electron in the ground state, compared with the first excited state (n=2) is A. B. C. D. E. ¼ as fast ½ as fast The same Twice as fast 4 times as fast 12
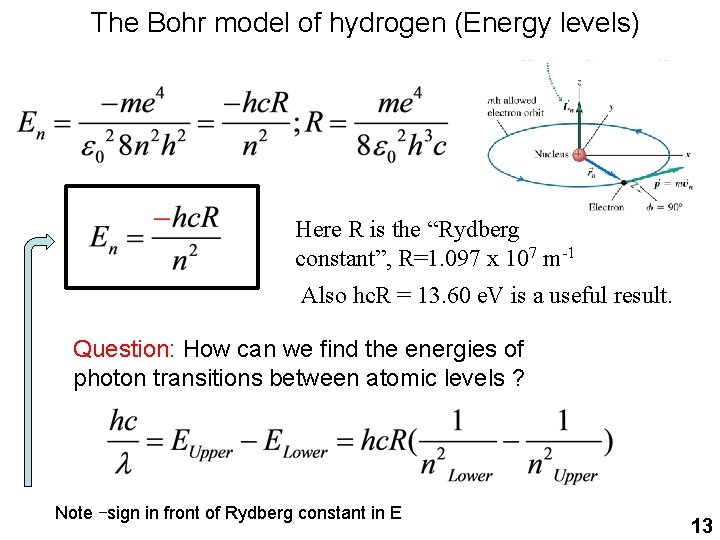
The Bohr model of hydrogen (Energy levels) Here R is the “Rydberg constant”, R=1. 097 x 107 m-1 Also hc. R = 13. 60 e. V is a useful result. Question: How can we find the energies of photon transitions between atomic levels ? Note –sign in front of Rydberg constant in E 13
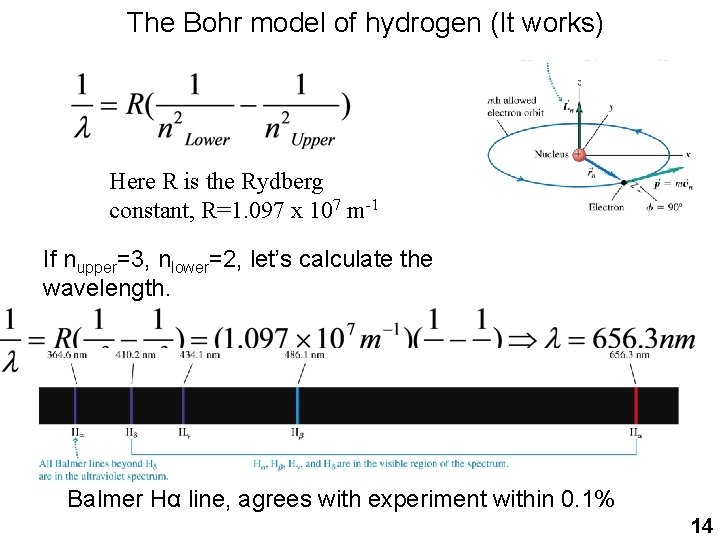
The Bohr model of hydrogen (It works) Here R is the Rydberg constant, R=1. 097 x 107 m-1 If nupper=3, nlower=2, let’s calculate the wavelength. Balmer Hα line, agrees with experiment within 0. 1% 14
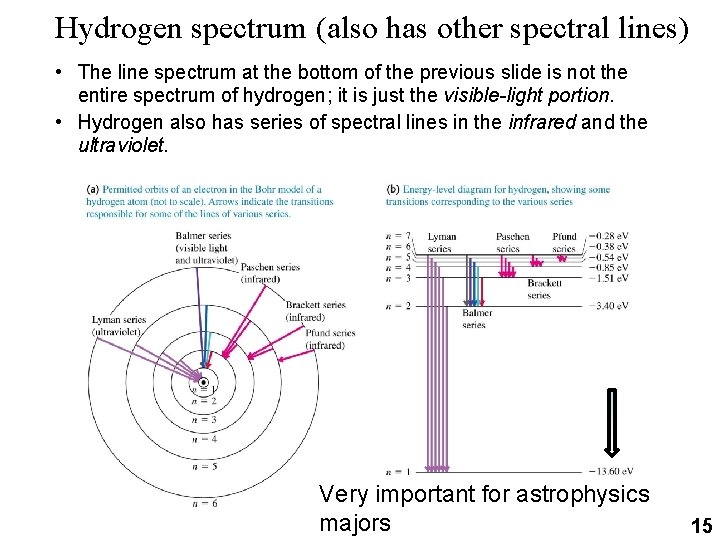
Hydrogen spectrum (also has other spectral lines) • The line spectrum at the bottom of the previous slide is not the entire spectrum of hydrogen; it is just the visible-light portion. • Hydrogen also has series of spectral lines in the infrared and the ultraviolet. Very important for astrophysics majors 15
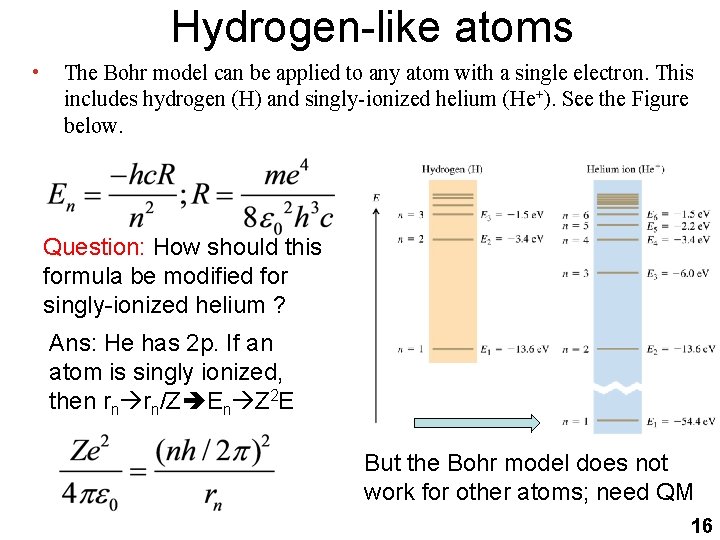
Hydrogen-like atoms • The Bohr model can be applied to any atom with a single electron. This includes hydrogen (H) and singly-ionized helium (He+). See the Figure below. Question: How should this formula be modified for singly-ionized helium ? Ans: He has 2 p. If an atom is singly ionized, then rn rn/Z En Z 2 E But the Bohr model does not work for other atoms; need QM 16
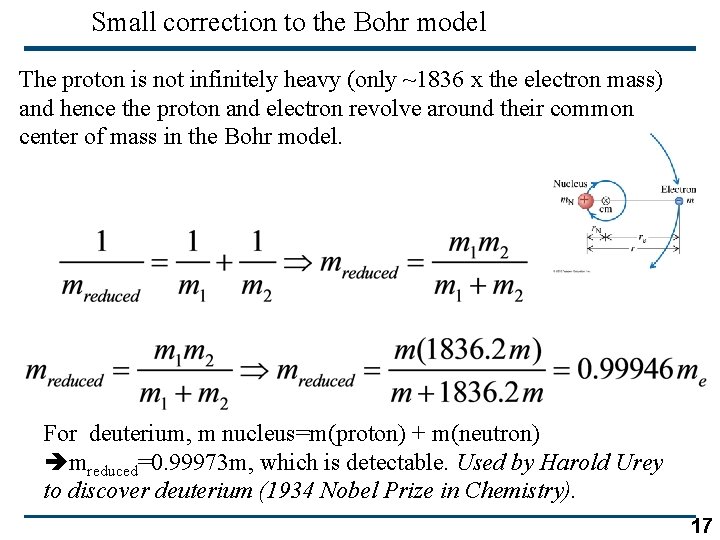
Small correction to the Bohr model The proton is not infinitely heavy (only ~1836 x the electron mass) and hence the proton and electron revolve around their common center of mass in the Bohr model. For deuterium, m nucleus=m(proton) + m(neutron) mreduced=0. 99973 m, which is detectable. Used by Harold Urey to discover deuterium (1934 Nobel Prize in Chemistry). 17

Review and Practice: The Bohr model of hydrogen (Clicker questions) 18
Q 20. 3 In the Bohr model of atom, what is the relationship between the radius of the electron orbit and the electron wavelength? 19
Q 20. 3 In the Bohr model of atom, what is the relationship between the radius of the electron orbit and the electron wavelength? 20

Q 20. 4 In the Bohr model of atom, the relationship the radius of the electron orbit and the electron wavelength is equivalent to A. B. C. D. E. Conservation of momentum Conservation of energy Conservation of angular momentum Spin Conservation Probability conservation 21
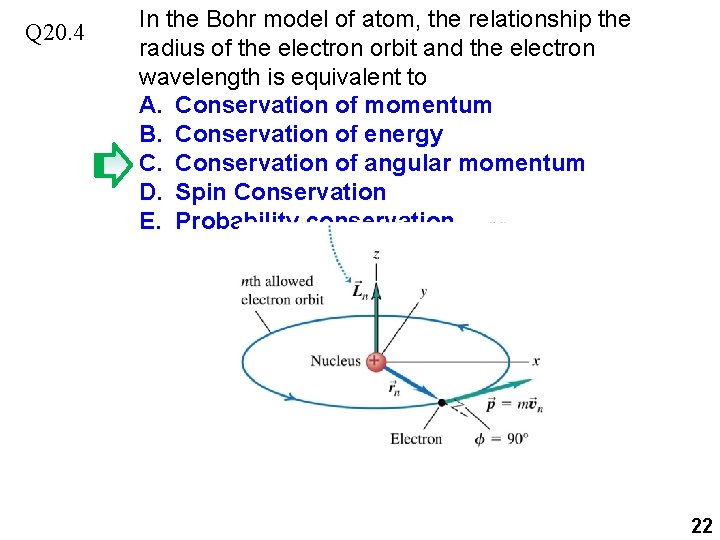
Q 20. 4 In the Bohr model of atom, the relationship the radius of the electron orbit and the electron wavelength is equivalent to A. Conservation of momentum B. Conservation of energy C. Conservation of angular momentum D. Spin Conservation E. Probability conservation 22
Q 20. 5 In the Bohr model of the hydrogen atom, the ground state orbit has a radius of 5. 3 x 10 -11 m. What is the radius of the first excited state orbit? A. B. C. D. 10. 6 x 10 -11 m 21. 2 x 10 -11 m 2. 65 x 10 -11 m 5. 3 x 10 -11 m 23

Q 20. 5 In the Bohr model of the hydrogen atom, the ground state orbit has a radius of 5. 3 x 10 -11 m. What is the radius of the first excited state orbit? A. B. C. D. 10. 6 x 10 -11 m 21. 2 x 10 -11 m 2. 65 x 10 -11 m 5. 3 x 10 -11 m Bohr model: r_n = n^2 a_0 B (x 4) 24
Application of the Bohr model In an alkali “Rydberg atom” the principal quantum number may reach n=1000. Question: How big is a Rydberg atom ? Still more clicker questions to come 25
Extra slides
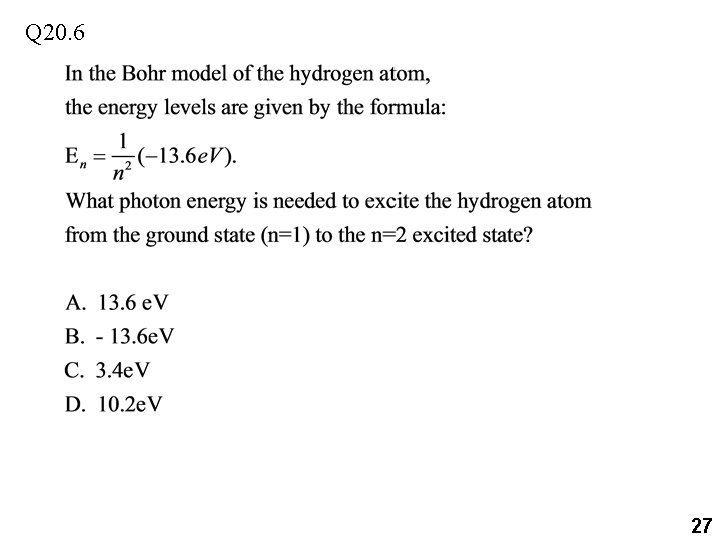
Q 20. 6 27
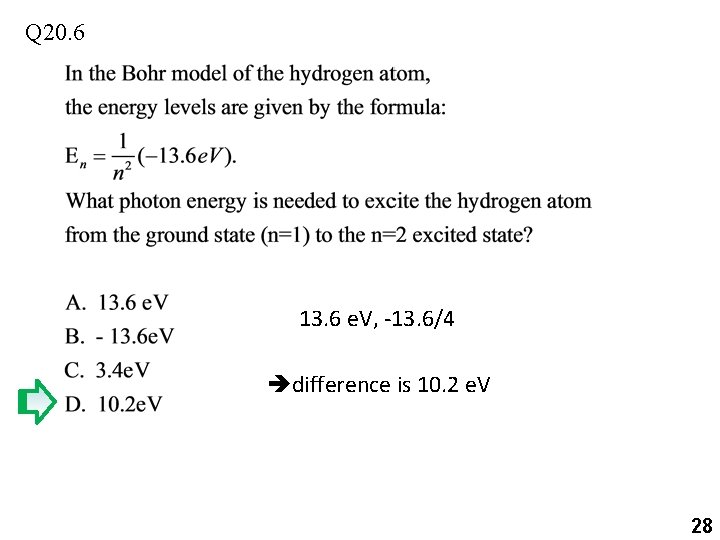
Q 20. 6 13. 6 e. V, -13. 6/4 difference is 10. 2 e. V 28
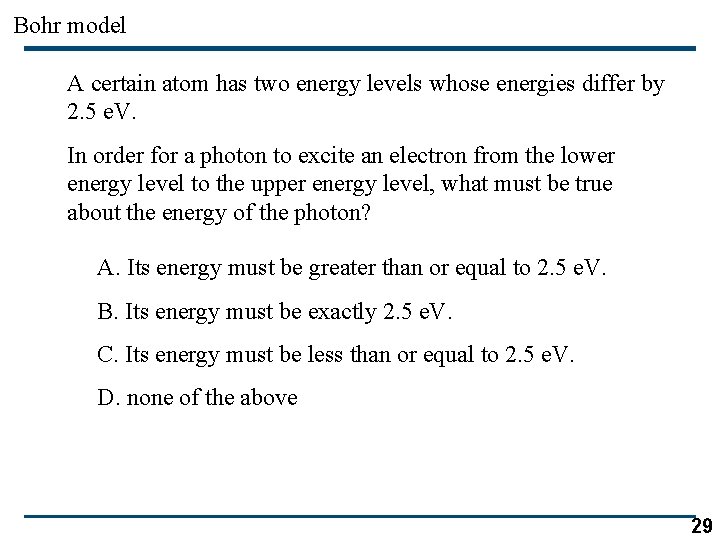
Bohr model A certain atom has two energy levels whose energies differ by 2. 5 e. V. In order for a photon to excite an electron from the lower energy level to the upper energy level, what must be true about the energy of the photon? A. Its energy must be greater than or equal to 2. 5 e. V. B. Its energy must be exactly 2. 5 e. V. C. Its energy must be less than or equal to 2. 5 e. V. D. none of the above 29
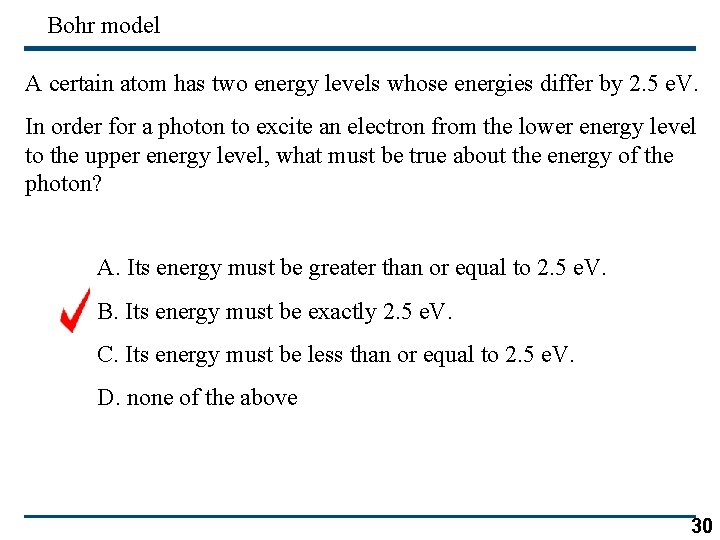
Bohr model A certain atom has two energy levels whose energies differ by 2. 5 e. V. In order for a photon to excite an electron from the lower energy level to the upper energy level, what must be true about the energy of the photon? A. Its energy must be greater than or equal to 2. 5 e. V. B. Its energy must be exactly 2. 5 e. V. C. Its energy must be less than or equal to 2. 5 e. V. D. none of the above 30
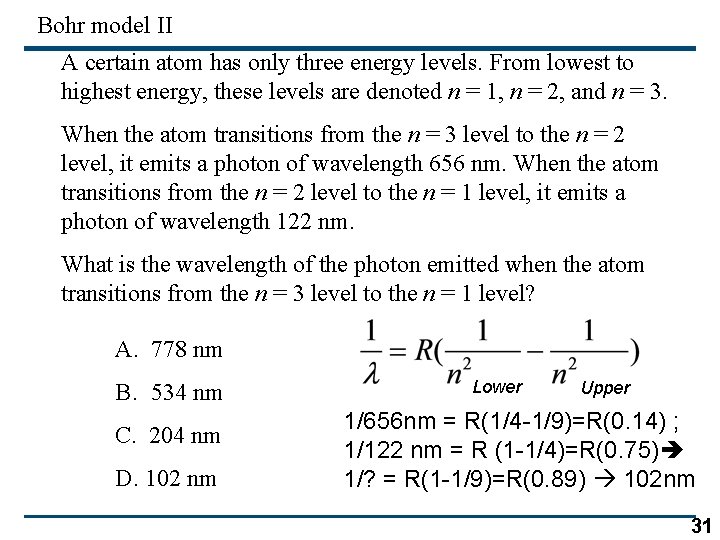
Bohr model II A certain atom has only three energy levels. From lowest to highest energy, these levels are denoted n = 1, n = 2, and n = 3. When the atom transitions from the n = 3 level to the n = 2 level, it emits a photon of wavelength 656 nm. When the atom transitions from the n = 2 level to the n = 1 level, it emits a photon of wavelength 122 nm. What is the wavelength of the photon emitted when the atom transitions from the n = 3 level to the n = 1 level? A. 778 nm B. 534 nm C. 204 nm D. 102 nm Lower Upper 1/656 nm = R(1/4 -1/9)=R(0. 14) ; 1/122 nm = R (1 -1/4)=R(0. 75) 1/? = R(1 -1/9)=R(0. 89) 102 nm 31
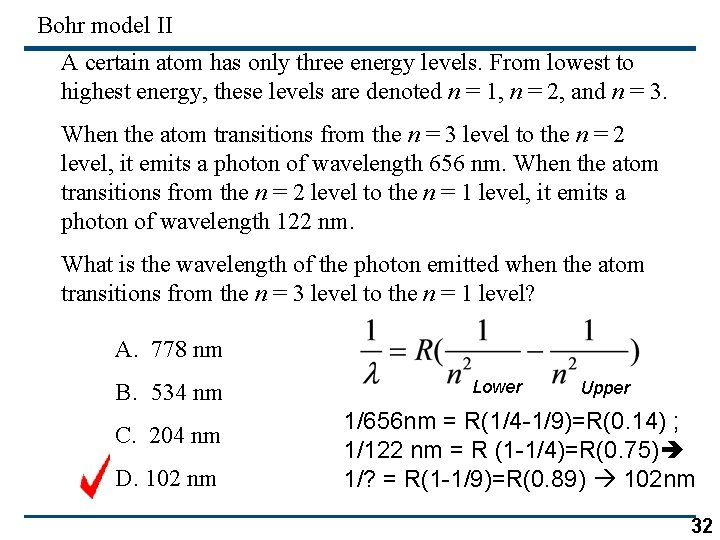
Bohr model II A certain atom has only three energy levels. From lowest to highest energy, these levels are denoted n = 1, n = 2, and n = 3. When the atom transitions from the n = 3 level to the n = 2 level, it emits a photon of wavelength 656 nm. When the atom transitions from the n = 2 level to the n = 1 level, it emits a photon of wavelength 122 nm. What is the wavelength of the photon emitted when the atom transitions from the n = 3 level to the n = 1 level? A. 778 nm B. 534 nm C. 204 nm D. 102 nm Lower Upper 1/656 nm = R(1/4 -1/9)=R(0. 14) ; 1/122 nm = R (1 -1/4)=R(0. 75) 1/? = R(1 -1/9)=R(0. 89) 102 nm 32
Bohr model III In the Bohr model of the hydrogen atom, an electron in the n = 2 orbit has A. a higher total energy and a higher kinetic energy than an electron in the n =1 orbit. B. a lower total energy and a higher kinetic energy than an electron in the n =1 orbit. C. a higher total energy and a lower kinetic energy than an electron in the n =1 orbit. D. a lower total energy and a lower kinetic energy than an electron in the n =1 orbit. E. none of the above 33
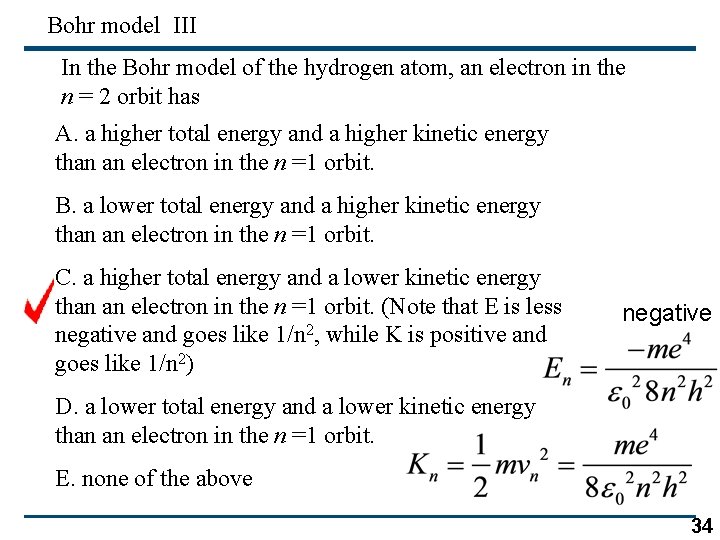
Bohr model III In the Bohr model of the hydrogen atom, an electron in the n = 2 orbit has A. a higher total energy and a higher kinetic energy than an electron in the n =1 orbit. B. a lower total energy and a higher kinetic energy than an electron in the n =1 orbit. C. a higher total energy and a lower kinetic energy than an electron in the n =1 orbit. (Note that E is less negative and goes like 1/n 2, while K is positive and goes like 1/n 2) negative D. a lower total energy and a lower kinetic energy than an electron in the n =1 orbit. E. none of the above 34
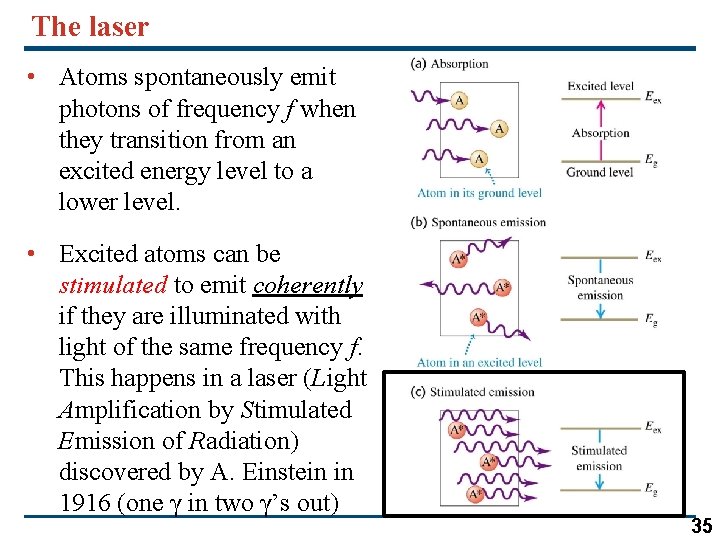
The laser • Atoms spontaneously emit photons of frequency f when they transition from an excited energy level to a lower level. • Excited atoms can be stimulated to emit coherently if they are illuminated with light of the same frequency f. This happens in a laser (Light Amplification by Stimulated Emission of Radiation) discovered by A. Einstein in 1916 (one γ in two γ’s out) 35
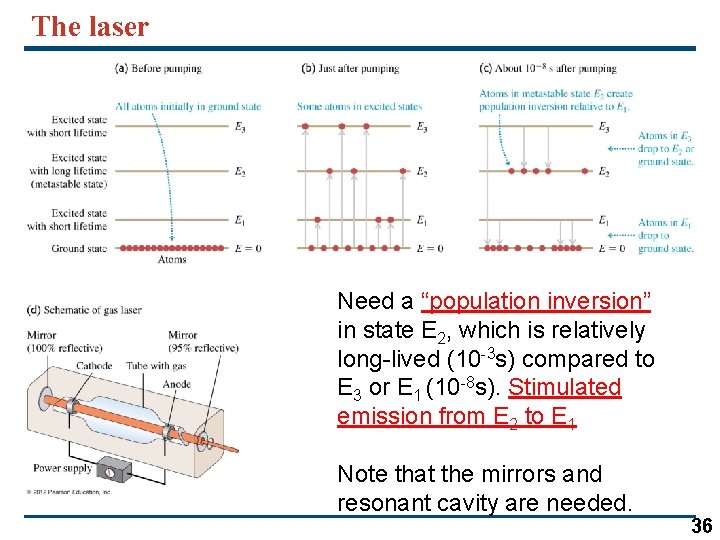
The laser Need a “population inversion” in state E 2, which is relatively long-lived (10 -3 s) compared to E 3 or E 1 (10 -8 s). Stimulated emission from E 2 to E 1 Note that the mirrors and resonant cavity are needed. 36
Conceptual question on lasers An ordinary neon light fixture like those used in advertising signs emit red light of wavelength 632. 8 nm. Neon is also used in a helium-neon laser (He. Ne). The light emitted by a neon light fixture is an example of A) Spontaneous emission B) Stimulated emission C) Both Spontaneous and stimulated emission. Ans: A (potential difference is applied across the tube to excite Ne atoms into an excited state). However, there is no population inversion or resonant cavity etc. 37
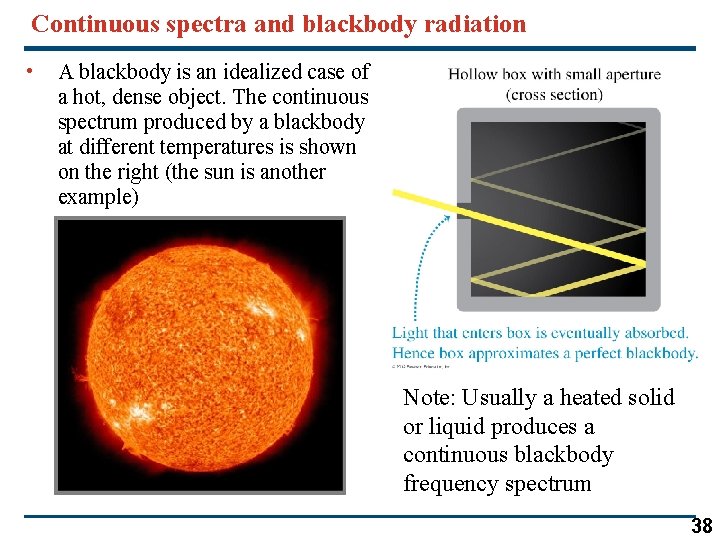
Continuous spectra and blackbody radiation • A blackbody is an idealized case of a hot, dense object. The continuous spectrum produced by a blackbody at different temperatures is shown on the right (the sun is another example) Note: Usually a heated solid or liquid produces a continuous blackbody frequency spectrum 38
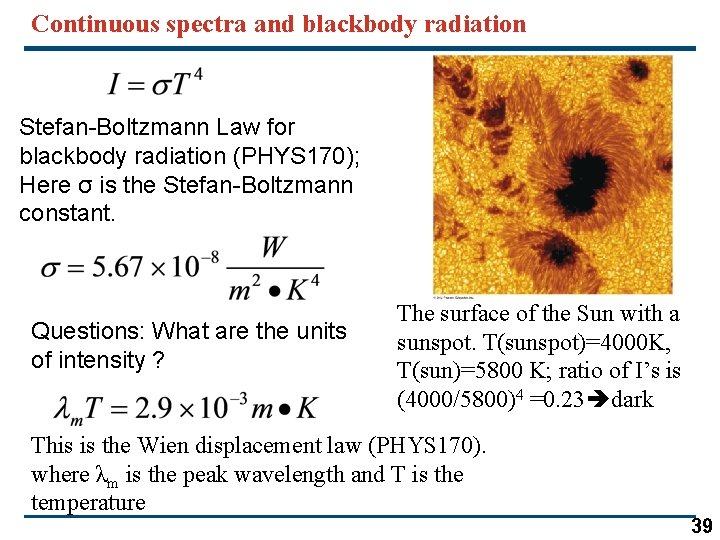
Continuous spectra and blackbody radiation Stefan-Boltzmann Law for blackbody radiation (PHYS 170); Here σ is the Stefan-Boltzmann constant. Questions: What are the units of intensity ? The surface of the Sun with a sunspot. T(sunspot)=4000 K, T(sun)=5800 K; ratio of I’s is (4000/5800)4 =0. 23 dark This is the Wien displacement law (PHYS 170). where λm is the peak wavelength and T is the temperature 39
- Slides: 39