QIAGEN Acquires Key Assets of Molecular Staging Inc










- Slides: 18

QIAGEN Acquires Key Assets of Molecular Staging, Inc. September 27, 2004 WWW. QIAGEN. COM

Welcome to QIAGEN‘s Conference Call QIAGEN Acquires Key Assets of Molecular Staging, Second Quarter and Half Year 2003 Conference Call Inc. September 27, 2004, 9: 30 am EST, 14: 30 GMT, 15: 30 MET Conference Call Outline: 1) 20 min Presentation Peer M. Schatz, CEO Roland Sackers, CFO Dr. Solveigh Mähler, Director IR 2) 40 min Q&A session Due to time restrictions we would like to ask for a maximum of TWO questions per caller. Slide: 2 WWW. QIAGEN. COM

Forward Looking Statements Certain of the statements contained in this presentation may be considered forward-looking statements within the meaning of Section 27 A of the U. S. Securities Act of 1933, as amended, and Section 21 E of the U. S. Securities Exchange Act of 1934, as amended. To the extent that any of the statements contained herein relating to QIAGEN's products and markets and operating results are forward-looking, such statements are based on current expectations that involve a number of uncertainties and risks. Such uncertainties and risks include, but are not limited to, risks associated with management of growth and international operations (including the effects of currency fluctuations), variability of operating results, the commercial development of the DNA sequencing, genomics and synthetic nucleic acid-related markets, as well as the nucleic acid-based molecular diagnostics and genetic vaccination and gene therapy markets, competition, rapid or unexpected changes in technologies, fluctuations in demand for QIAGEN's products (including seasonal fluctuations), difficulties in successfully adapting QIAGEN’s products to integrated solutions and producing such products, the ability of QIAGEN to identify and develop new products and to differentiate its products from competitors, and the integration of acquisitions of technologies and businesses. For further information, refer to the discussion in reports that QIAGEN has filed with the U. S. Securities and Exchange Commission (SEC). Slide: 3 WWW. QIAGEN. COM

Our Mission: Transaction Adds Technology in Core Focus Area QIAGEN is the market and technology leader in nucleic acid sample handling, separation and purification. Our mission is to provide an outstanding contribution to our customers’ success by innovating and supplying our products and services in all areas where they require this expertise. Our products and technologies enable our customers to achieve breakthroughs in research and new standards in healthcare which both contribute to improving lives. By focusing on increasing our customers’ success, the exceptional talent and commitment of our employees bring excellence to all segments of the value chain and outstanding success to QIAGEN. Slide: 4 WWW. QIAGEN. COM

Transaction Rationales • Highly core to QIAGEN: • Nucleic acid sample handling • Adding emerging and key segment to core • Highly synergistic: • Same customer base • Same S&M approach • Overlapping applications • Same product formats • Huge patent estate: approx. 160 patents • Accretive in 2005: • Adds $6 million in sales • Adds $1 million in net income • Adds strong basis for fast growth • Fast integration planned – completed by the end of 2004 Slide: 5 WWW. QIAGEN. COM

DNA Constraint is a Major Problem & Market Opportunity • Increasing number of tests and analyses requires larger amounts of genomic DNA • • Genome-wide SNP analyses Sharing of clinical samples across disease panels / locations Patient genotyping Re-analysis at later time • The amount of sample material is often constrained • Scarce or limited samples – tissue banks, plant and animal collections, small needle biopsies, clinical trial samples • Pressure to make collection of sample less intrusive or simpler (buccal swabs etc. ) limits collected sample volume • Nanotechnologies and microfluidics put limitations on sample volumes Slide: 6 WWW. QIAGEN. COM

Whole Genome Amplification n “I want to have other labs run tests on this DNA sample” n “I want to do more tests using this DNA” n “The collection of these samples was very expensive” n “We can’t go back to the patients to collect more samples” SOLUTION: WGA Eliminating Sample Limitations Slide: 7 WWW. QIAGEN. COM

Highly Synergistic: Same Customer Base Clinical Research Market Molecular Diagnostics § § § § Genetic databases Clinical samples (e. g. small needle biopsies) DNA banking Very small amount of starting material Precious samples Irretrievable samples Genome wide studies Customers want MORE DNA out of LESS sample. Slide: 8 WWW. QIAGEN. COM

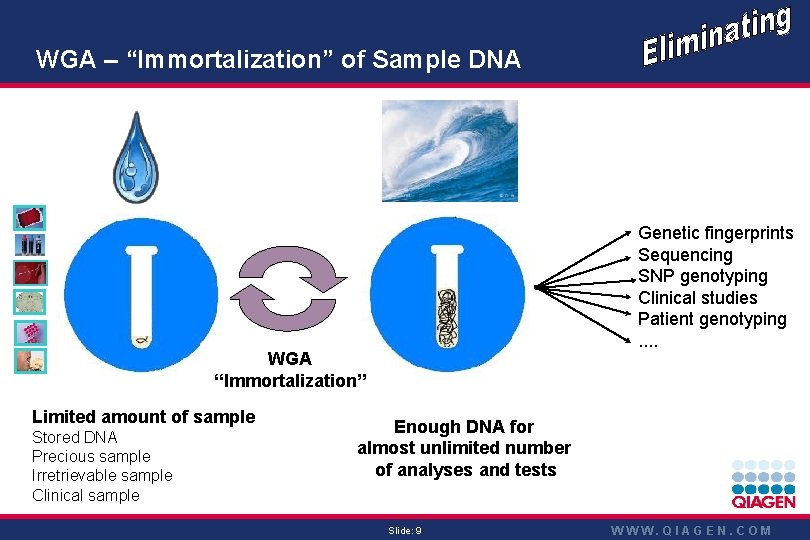
WGA – “Immortalization” of Sample DNA Genetic fingerprints Sequencing SNP genotyping Clinical studies Patient genotyping. . WGA “Immortalization” Limited amount of sample Stored DNA Precious sample Irretrievable sample Clinical sample Enough DNA for almost unlimited number of analyses and tests Slide: 9 WWW. QIAGEN. COM

Adding a Solution for an Emerging Need in our Core Focus Area Collection Stabilization Very small amounts of sample Clinical samples (e. g. small needle biopsies) Purification WGA “Immortalization” Create more DNA in Sample Limited amount of sample Precious and irretrievable samples Genome wide studies Slide: 10 Assay (e. g. PCR) Detect “Unlimited” Number of Analyses Genetic fingerprinting Sequencing SNP genotyping Clinical studies Patient genotyping. . WWW. QIAGEN. COM

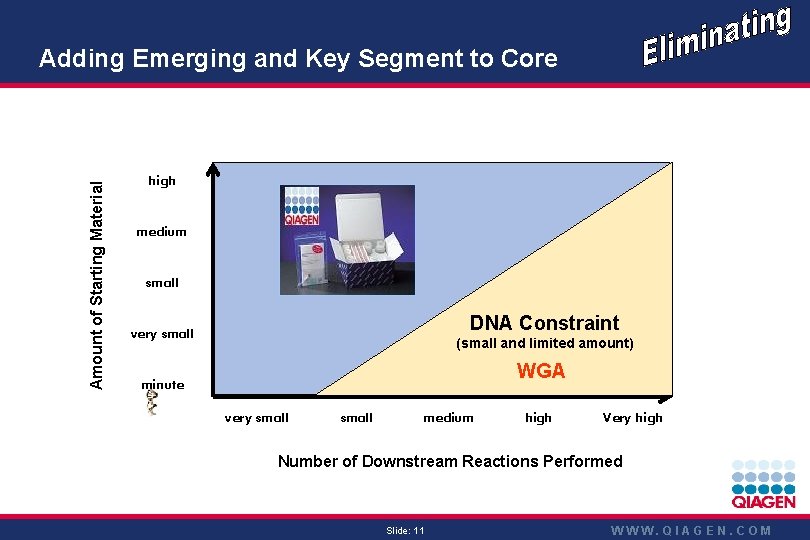
Amount of Starting Material Adding Emerging and Key Segment to Core high QIAamp DNeasy Q-Genomic medium small DNA Constraint very small (small and limited amount) WGA minute very small medium high Very high Number of Downstream Reactions Performed Slide: 11 WWW. QIAGEN. COM

DNA Constraint. Overlapping Samples Highly Synergistic: Applications Amount of DNA Provided by mg Whole Blood Buccal Swabs DNA Constraint Immortalization µg Dried Blood (Have sample, but need more DNA because I want to run many analyses) Serum Plasma FNA Biopsy ng DNA Constraint Micro (Have not much sample, Need more DNA to analyze Sample) LMD Tissue Case Work Samples pg pg Single PCR ng Multi PCR µg SNP Genotyping mg Amount of DNA Needed for Slide: 12 WWW. QIAGEN. COM

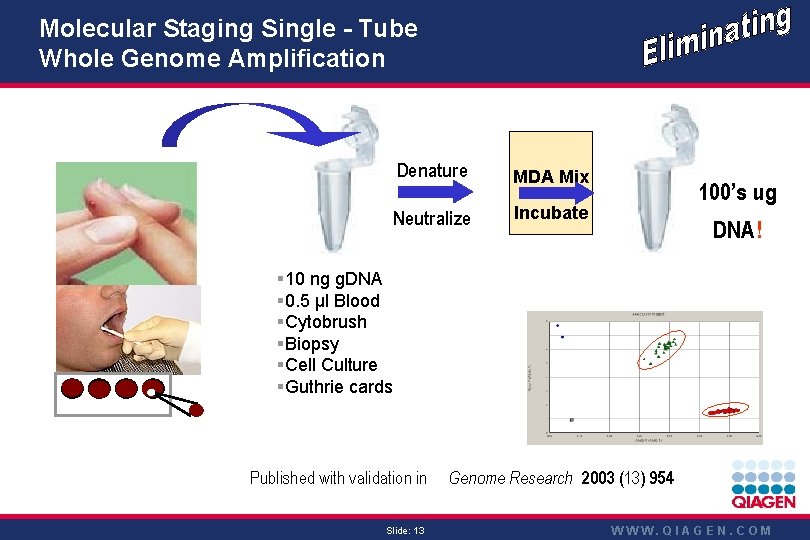
Molecular Staging Single - Tube Whole Genome Amplification Denature MDA Mix Neutralize Incubate 100’s ug DNA! § 10 ng g. DNA § 0. 5 µl Blood §Cytobrush §Biopsy §Cell Culture §Guthrie cards Published with validation in Slide: 13 Genome Research 2003 (13) 954 WWW. QIAGEN. COM

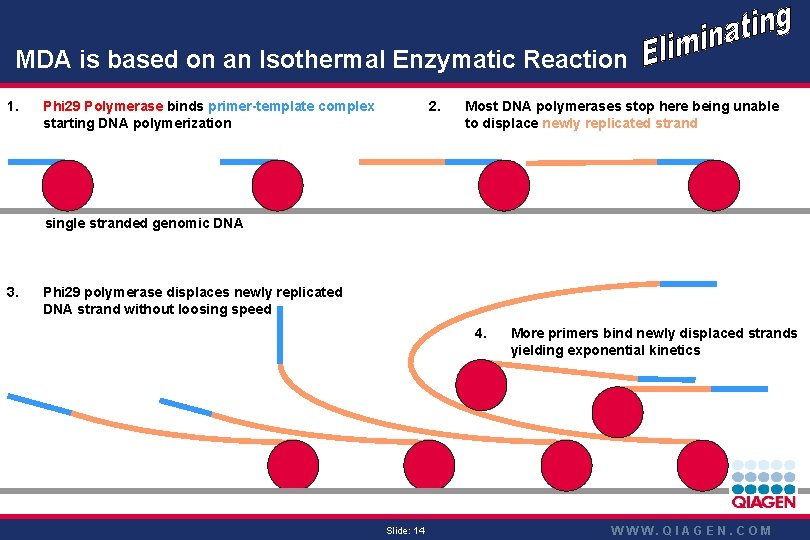
Phi 29 -DNA Pol mediated SDA using random MDA is based onprimers an Isothermal Enzymatic Reaction 1. 2. Phi 29 Polymerase binds primer-template complex starting DNA polymerization Most DNA polymerases stop here being unable to displace newly replicated strand single stranded genomic DNA 3. Phi 29 polymerase displaces newly replicated DNA strand without loosing speed 4. Slide: 14 More primers bind newly displaced strands yielding exponential kinetics WWW. QIAGEN. COM

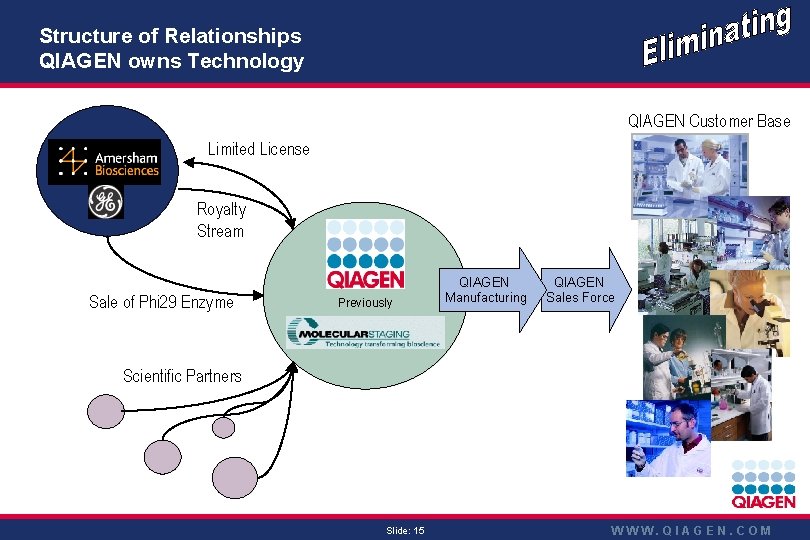
Structure of Relationships QIAGEN owns Technology QIAGEN Customer Base Limited License Royalty Stream Sale of Phi 29 Enzyme Previously QIAGEN Manufacturing QIAGEN Sales Force Scientific Partners Slide: 15 WWW. QIAGEN. COM

Executive Summary • Acquired the key assets of Molecular Staging, Inc. • MDA – the leading Whole Genome Amplification (WGA) • • technology Products and service business for nucleic acids Prestigious customer base Royalty stream from GEHB (Amersham) Proteomics technology for all downstream applications • Addressing the dynamic and core market of DNA-constraint • Highly synergistic to QIAGEN’s customer base with overlapping applications, same product formats and same S&M approach Slide: 16 WWW. QIAGEN. COM

Executive Summary • $28. 5 million in cash plus up to $6. 75 million sales-milestones • QIAGEN expects to incur one-time charges relating to this acquisition of approx. $2 million in Q 3 2004 • Positive financial impact • Accretive to 2005 EPS and adds higher growth rate • Expected to add $6 million in sales and $1 million in net income in 2005 • Integration into QIAGEN facilities and processes by end of 2004 • Transfer of operations to Germantown, MD and Hilden, Germany • Transfer of R&D to Hilden, Germany, further expansion planned • Worldwide Technical Service in Valencia, CA and Hilden, Germany Slide: 17 WWW. QIAGEN. COM

Guidance § 2005: § Standard WGA and RCA-based products begin marketing § Slightly Accretive to 2005 EPS § Adds $6 million in sales and $1 million in net income § 2006 and beyond § Combinations with QIAGEN products and technologies begin marketing § Rapid growth in revenues and profitability Slide: 18 WWW. QIAGEN. COM