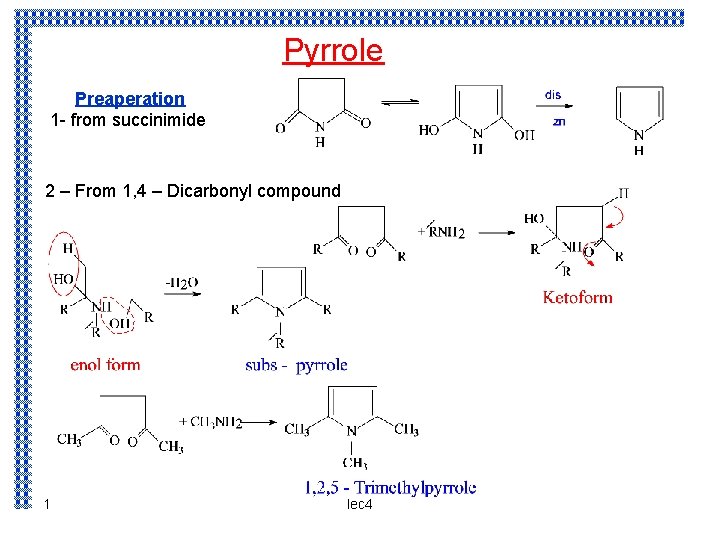
Pyrrole Preaperation 1 from succinimide 2 From 1


- Slides: 10

Pyrrole Preaperation 1 - from succinimide 2 – From 1, 4 – Dicarbonyl compound 1 lec 4

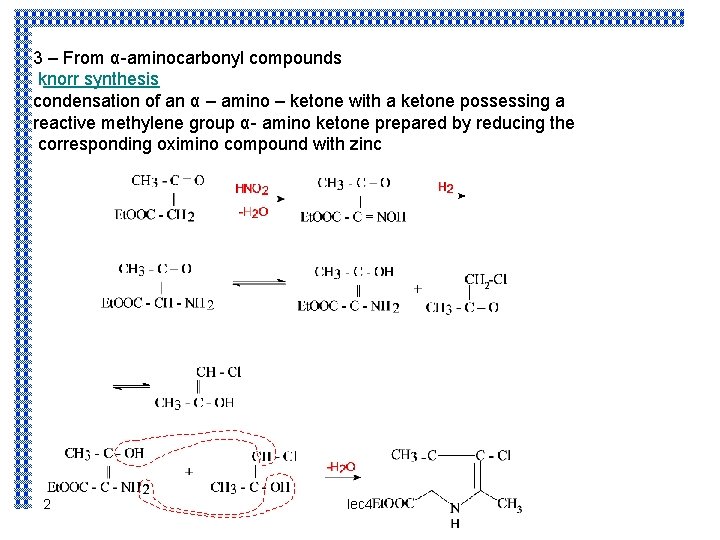
3 – From α-aminocarbonyl compounds knorr synthesis condensation of an α – amino – ketone with a ketone possessing a reactive methylene group α- amino ketone prepared by reducing the corresponding oximino compound with zinc 2 lec 4

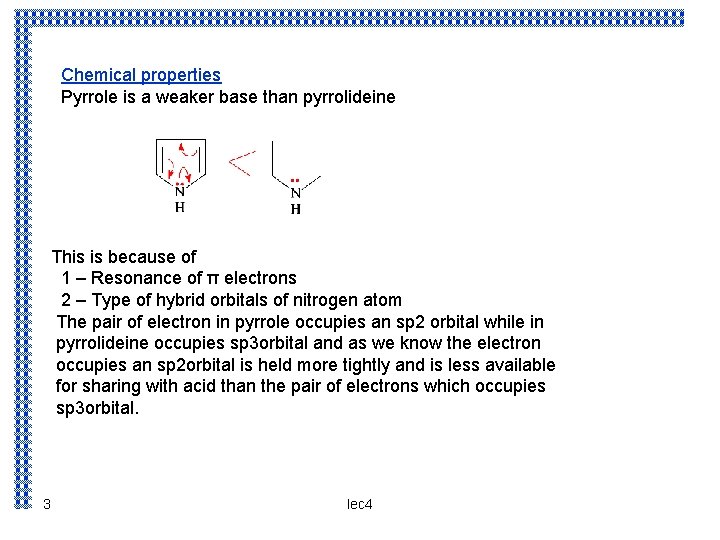
Chemical properties Pyrrole is a weaker base than pyrrolideine This is because of 1 – Resonance of π electrons 2 – Type of hybrid orbitals of nitrogen atom The pair of electron in pyrrole occupies an sp 2 orbital while in pyrrolideine occupies sp 3 orbital and as we know the electron occupies an sp 2 orbital is held more tightly and is less available for sharing with acid than the pair of electrons which occupies sp 3 orbital. 3 lec 4

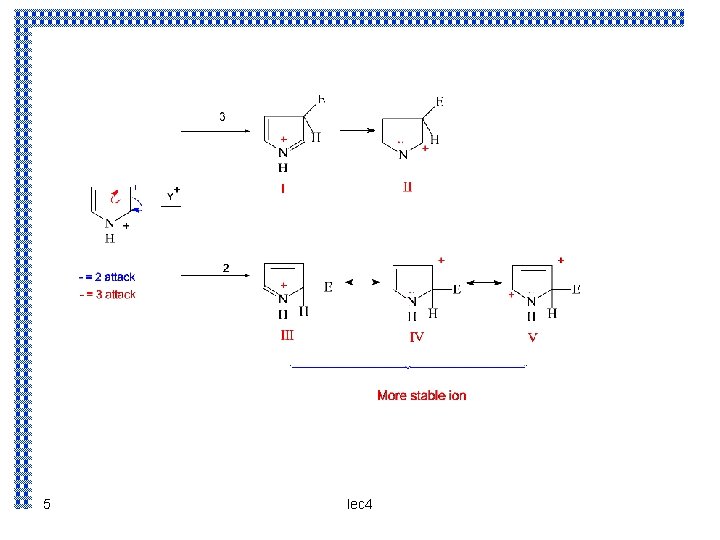
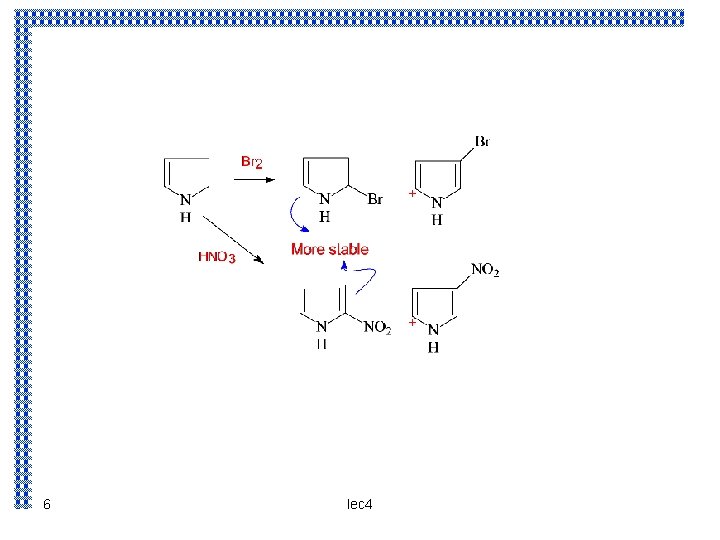
Electrophilic subistitution : The reaction takes place predominantly at the α – position we could account for this orientation of the following basis : the controlling step is the attachment of the electrophilic reagent which takes place in such a way as to yield the most stable intermediate carbonium ion Attack at position 3 yields carbonium ion that is a hybrid of structure I and II. Attack at position 2 yields a carbonium ion that is a hybrid not only of structure III and IV ( analogous ) to (I and II ) but also of structure V ; the extra stabilization conferred by V makes this ion the more stable one 4 lec 4

5 lec 4

+ + 6 lec 4

Furan Synthesis 1 – Feist Benary synthesis Treating α – chloroketone with ethyl acetoacetate CH 3 - C - OH || CH - Cl + HO CH - OOCEt || CH - CH 3 -HCl CH 3 - C -H 2 O HC Pyridine C -OOCEt O C - CH 3 2, 4 - di methyl-3 -ethylcarbonyl furan 7 lec 4

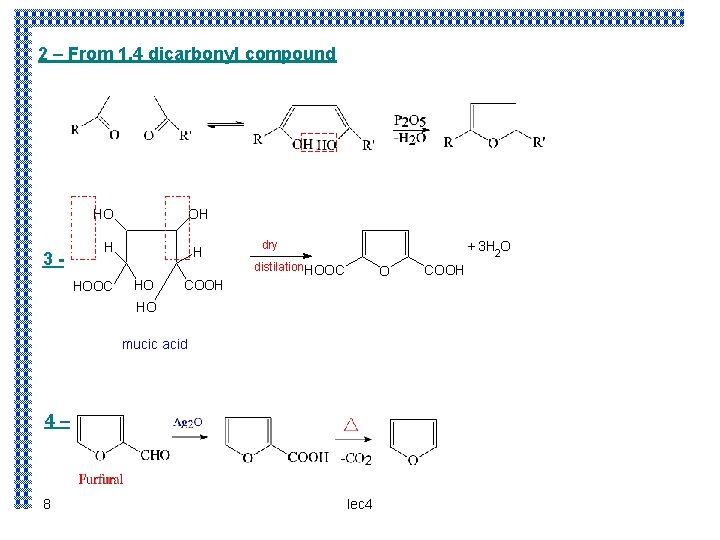
2 – From 1, 4 dicarbonyl compound HO 3 - OH H HOOC H HO COOH dry + 3 H 2 O distilation HOOC O HO mucic acid 4– 8 lec 4 COOH

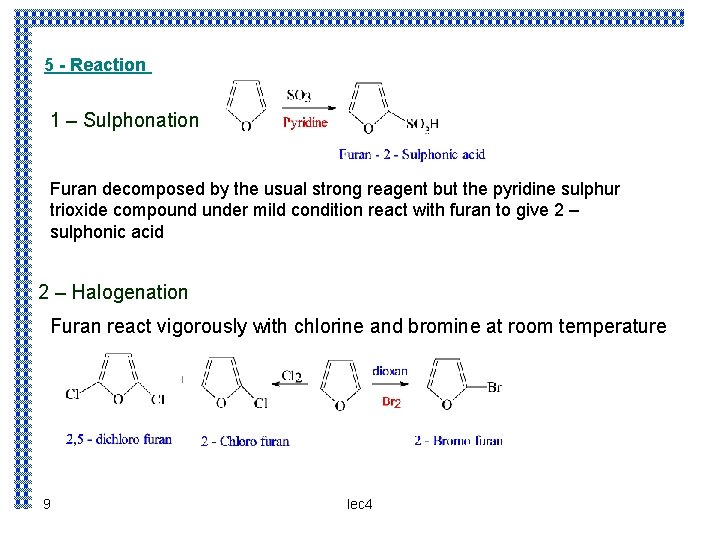
5 - Reaction 1 – Sulphonation Furan decomposed by the usual strong reagent but the pyridine sulphur trioxide compound under mild condition react with furan to give 2 – sulphonic acid 2 – Halogenation Furan react vigorously with chlorine and bromine at room temperature 9 lec 4

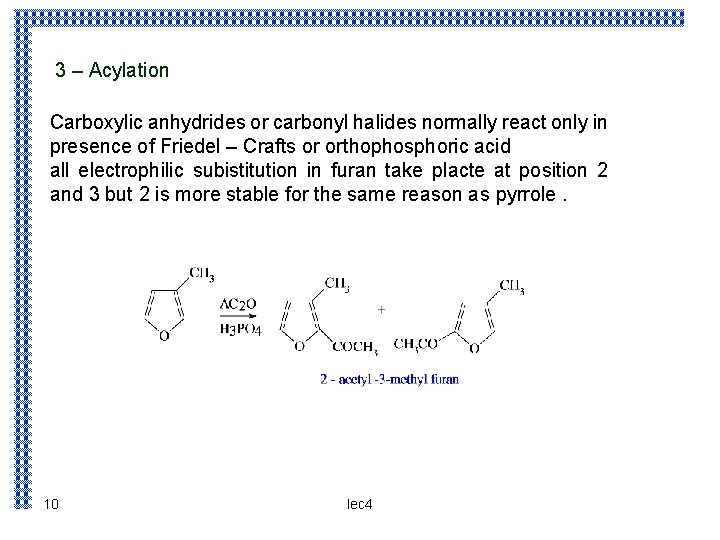
3 – Acylation Carboxylic anhydrides or carbonyl halides normally react only in presence of Friedel – Crafts or orthophosphoric acid all electrophilic subistitution in furan take placte at position 2 and 3 but 2 is more stable for the same reason as pyrrole. 10 lec 4