PYROLYSIS OF ACETALDEHYDE A FLEETING GLIMPSE OF VIN










- Slides: 26

PYROLYSIS OF ACETALDEHYDE: A FLEETING GLIMPSE OF VIN Why do this? The experiments The products The mechanism

PYROLYSIS OF ACETALDEHYDE: A FLEETING GLIMPSE OF VIN Senior Participants: M. Ahmed, J. R. Barker, J. W. Daily, M. R. Nimlos, D. L. Osborn, J. F. Stanton Postdocs and Students: Lawrence Berkeley Laboratory University of Michigan University of Colorado National Renewable Energy Lab Combustion Res. Facility, Sandia University of Texas A. Golan, L. Nguyen, M. Harding, O. Kostko, C. Miller*, B. Mc. Kown*, K. Piech, D. Tabor*, A. Vasilou and……. . A. *Undergraduates!

Our team leader

Why Biomass? Plants grow & pull CO 2 from the atmosphere. Burning the biomass (combustion) to CO 2 + H 2 O energy Returns CO 2 to the atmosphere: Carbon neutral (on “short” timescale) • Bio-refining: “grass, sticks” + H 2 + CO (syngas) CH 3 CH 2 OH Horrifically inefficient ! • Atmospheric Problem? Forest fires (air) + biomass aerosols


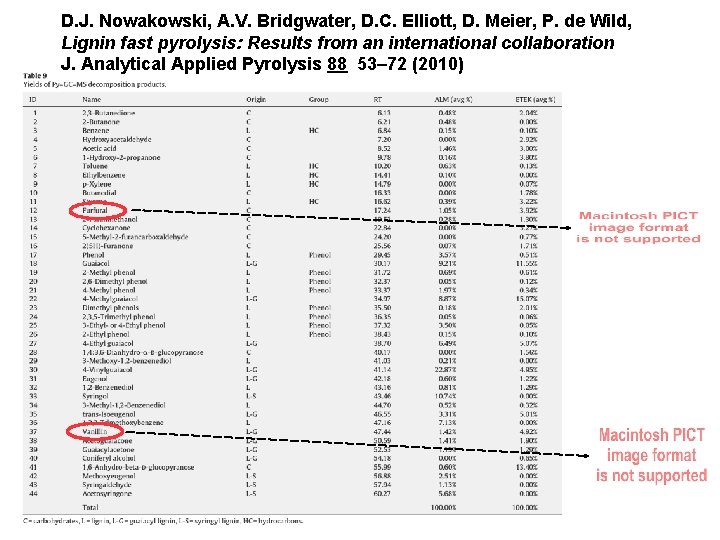
D. J. Nowakowski, A. V. Bridgwater, D. C. Elliott, D. Meier, P. de Wild, Lignin fast pyrolysis: Results from an international collaboration J. Analytical Applied Pyrolysis 88 53– 72 (2010)

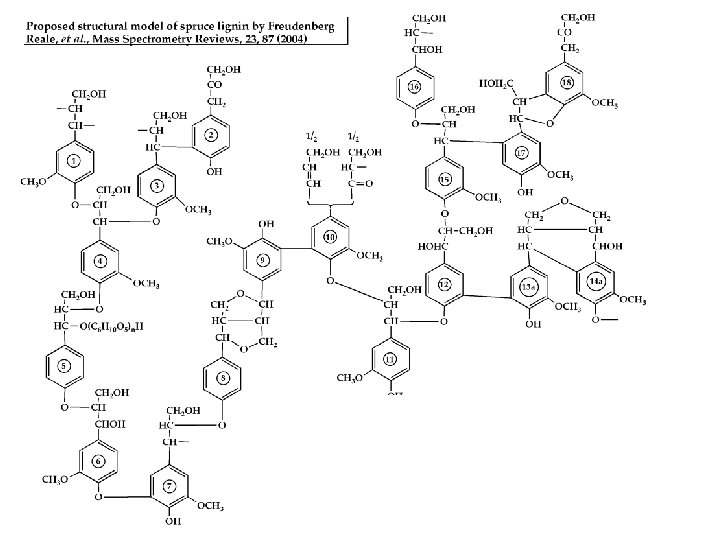
Carbohydrates (cellulose) important biomass component

TGA analysis J. P. Diebold, "A Unified, Global-Model for the Pyrolysis of Cellulose. " Biomass & Bioenergy 7 7585 (1994) Phenomenological kinetics/rapid heating to 500º C J W Daily

Let’s start with something simple Aldehydes are involved , and the simplest organic aldehyde is acetaldehyde: CH 3 CHO + ∆ products (monitor by PIMS and IR) What are the bond energies of CH 3 CHO ? Most (all? ) studies have focused on the upper pathway; other decomposition routes unknown/unexplored.

Chen nozzle (aka “tubular reactor”) Si. C tube, walls resistively heated to T up to ca. 1500 K

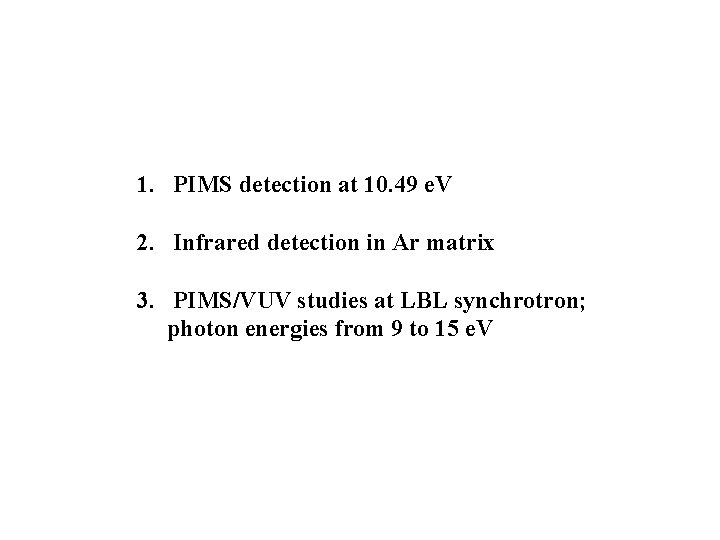
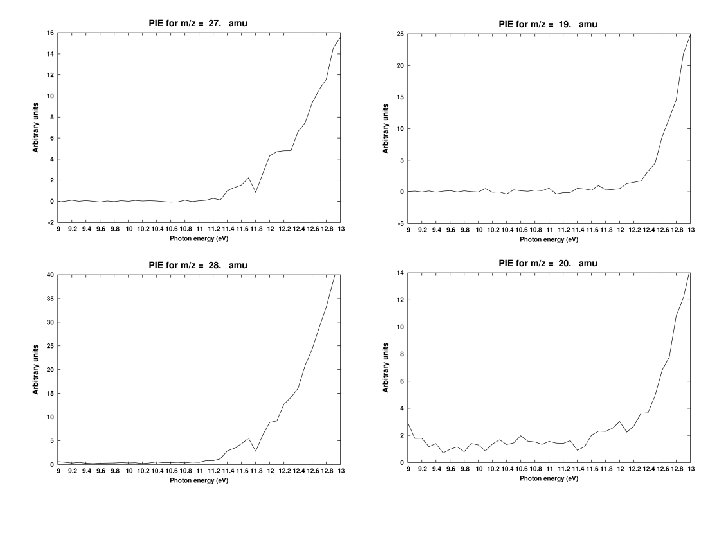
1. PIMS detection at 10. 49 e. V 2. Infrared detection in Ar matrix 3. PIMS/VUV studies at LBL synchrotron; photon energies from 9 to 15 e. V

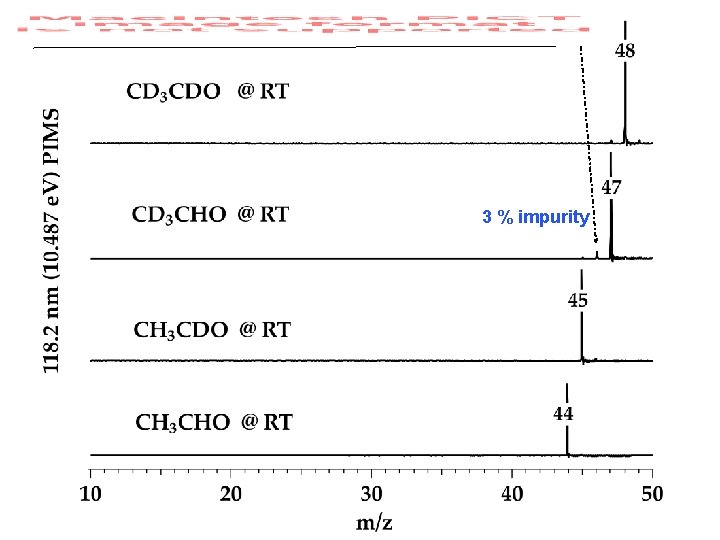
3 % impurity

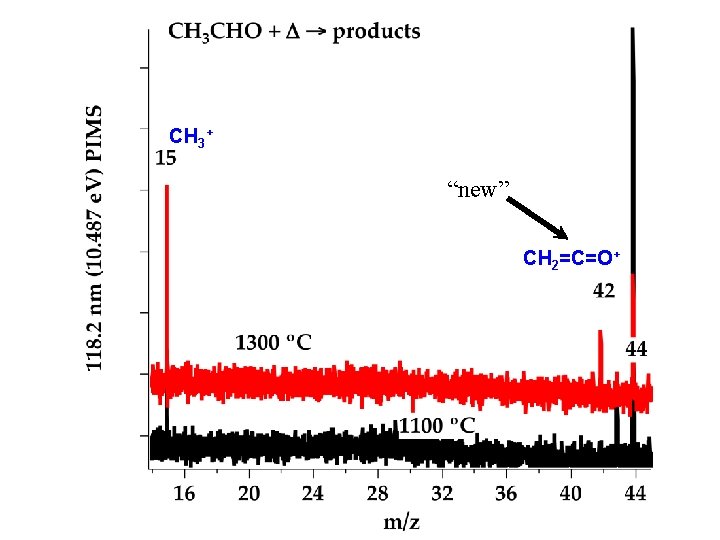
CH 3+ “new” CH 2=C=O+

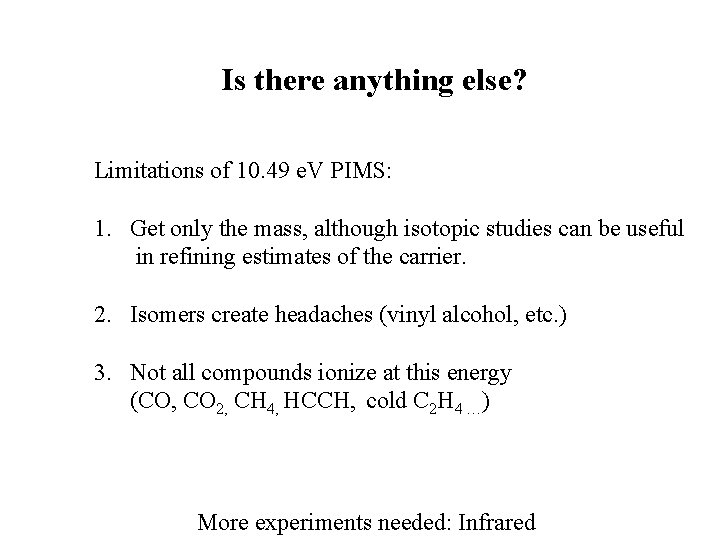
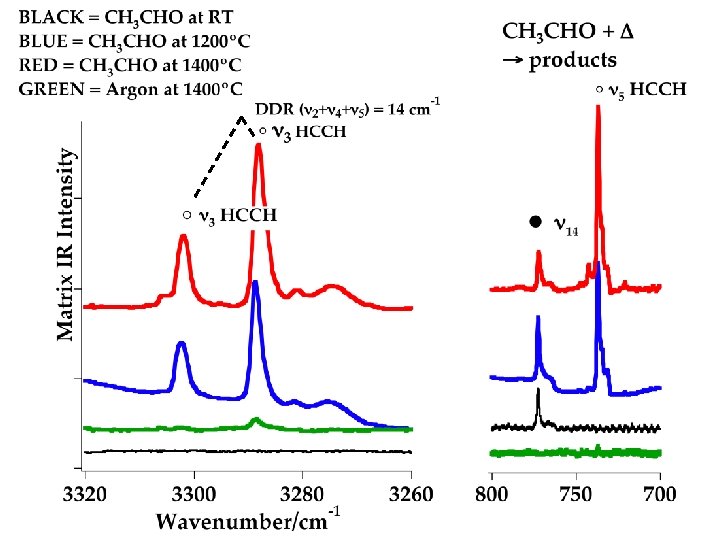
Is there anything else? Limitations of 10. 49 e. V PIMS: 1. Get only the mass, although isotopic studies can be useful in refining estimates of the carrier. 2. Isomers create headaches (vinyl alcohol, etc. ) 3. Not all compounds ionize at this energy (CO, CO 2, CH 4, HCCH, cold C 2 H 4 …) More experiments needed: Infrared


Acetylene! See talk by R. W. Field, MSS, 2009. Not immediately obvious where this comes from.



Mechanism(s)? Limitations of matrix IR: 1. Very hard to discriminate some isotopic species. 2. Tremendous amount of spectral congestion complicates search for new compounds (transients). Theorists sometimes needed to estimate line positions. More experiments needed: ALS synchrotron

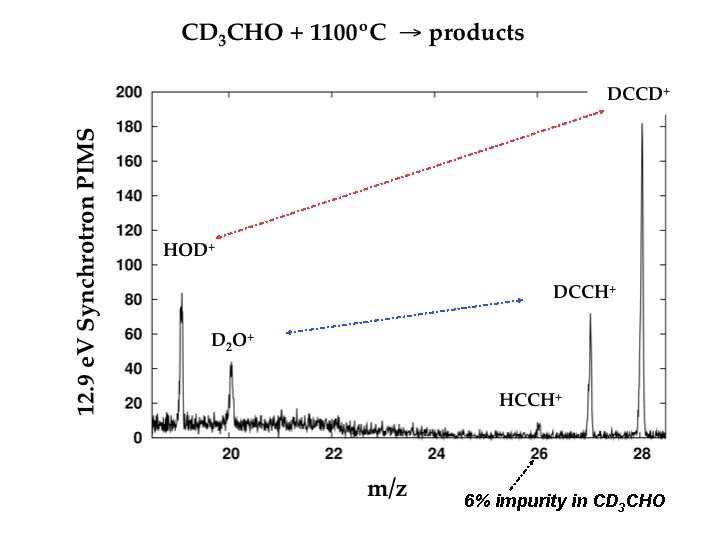
6% impurity in CD 3 CHO


Supported by additional isotopic studies, calculations with semiclassical transition state theory (SCTSTa) See: W. H. Miller JCP 62, 1899 (1975); T. L. Nguyen, JFS and J. R. Barker JCP A 115, 5118 (2011).

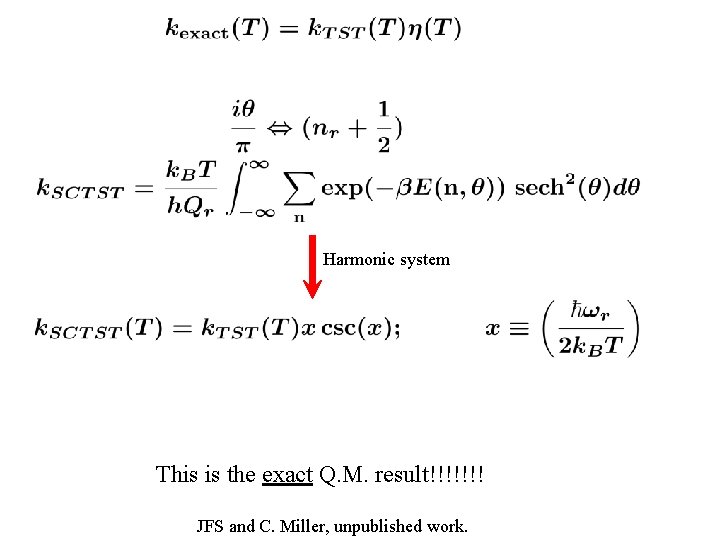
Harmonic system This is the exact Q. M. result!!!!!!! JFS and C. Miller, unpublished work.

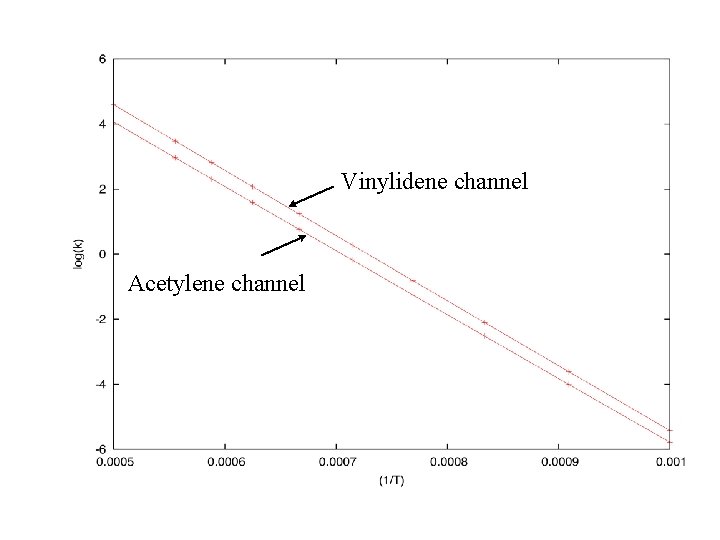
Vinylidene channel Acetylene channel

Conclusions Thermal pyrolysis of acetaldehyde is more complicated than commonly believed. Ketene, acetylene and water are “new” closed-shell (stable) products. Isomerization to vinyl alcohol is competitive with, and perhaps faster than, decomposition to methyl and CO. Isotopic evidence shows clearly that a great deal of vinyl alcohol dehydration involves vinylidene as an intermediate. There is a role for theory in “biomass science”

The end