px p z x 2 p orbitals y





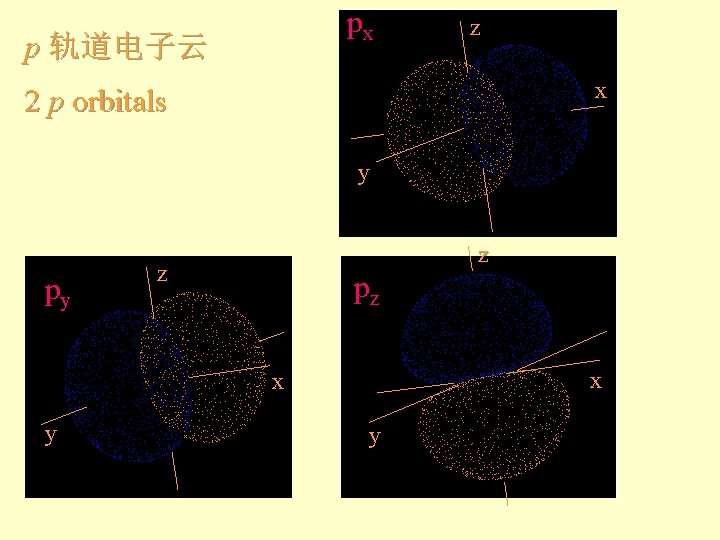
px p 轨道电子云 z x 2 p orbitals y py z z pz x x y y
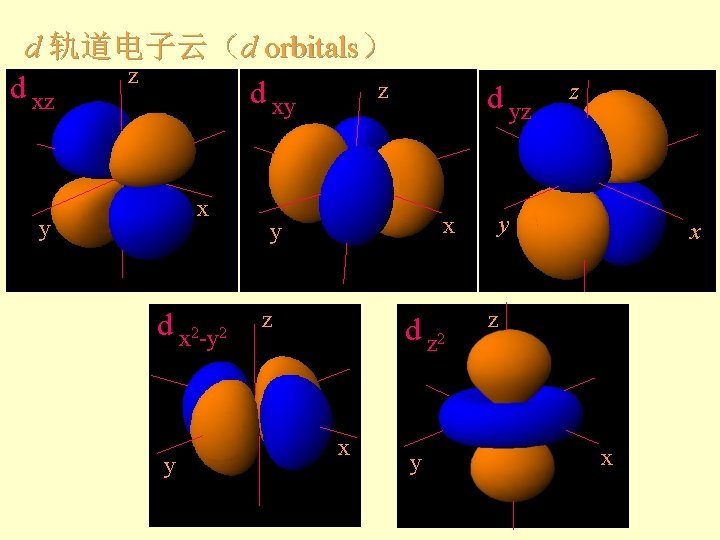
d 轨道电子云(d orbitals) z d xz z d xy x y d x 2 -y 2 y d yz x y z d z 2 x y z y x z x

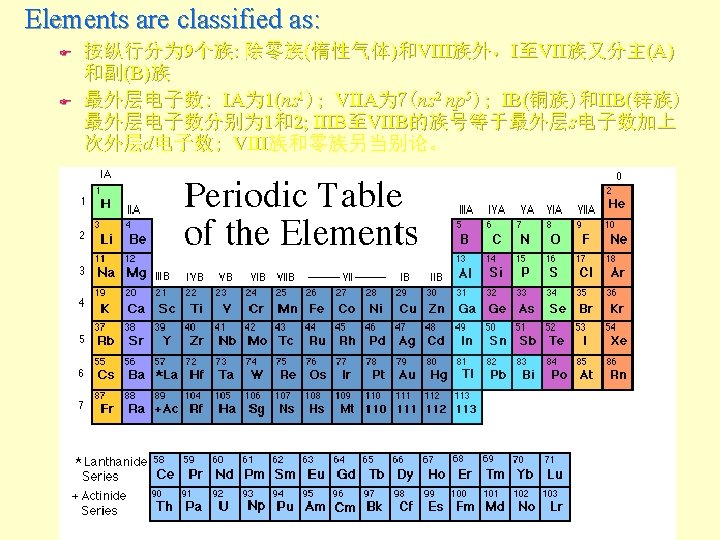
The Periodic Table
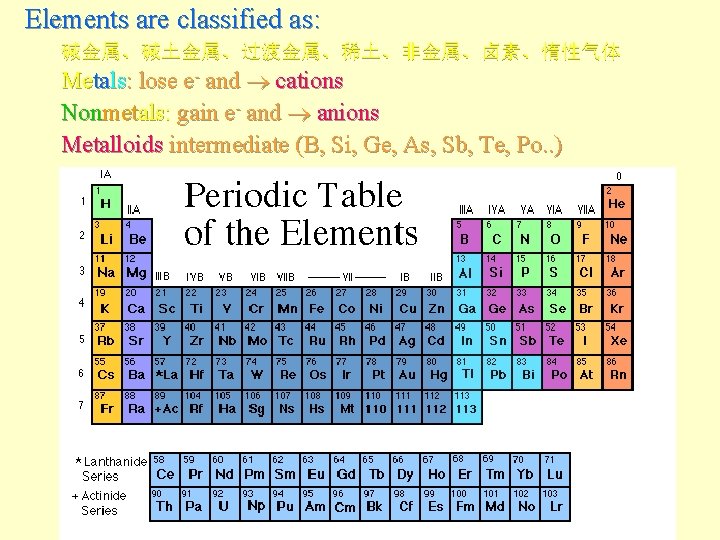
Elements are classified as: 碱金属、碱土金属、过渡金属、稀土、非金属、卤素、惰性气体 Metals: lose e- and cations Nonmetals: gain e- and anions Metalloids intermediate (B, Si, Ge, As, Sb, Te, Po. . )

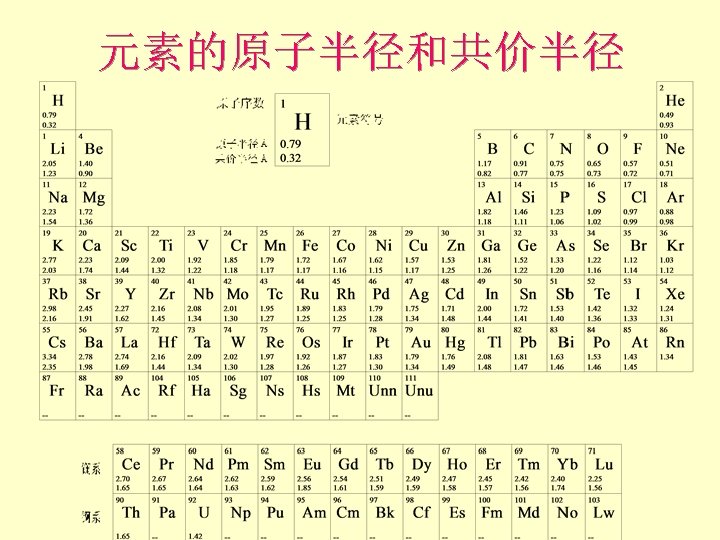
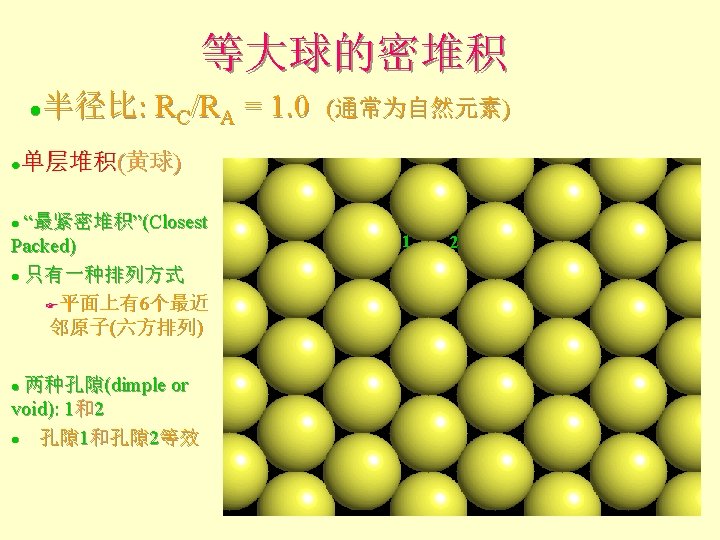
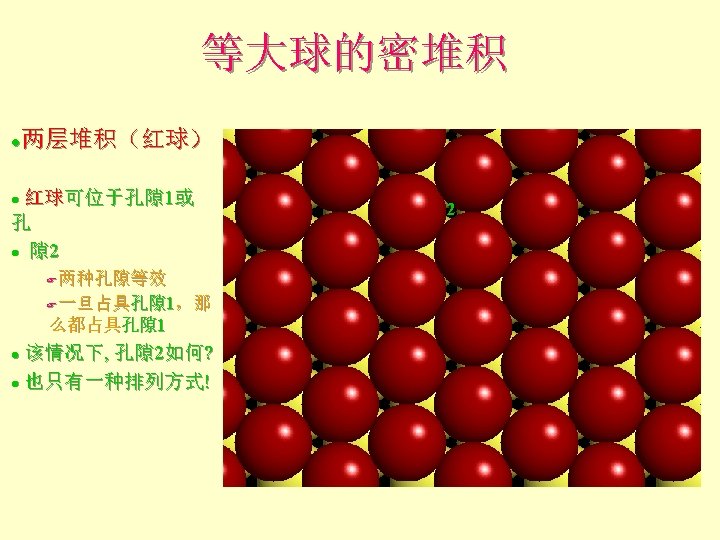
Atomic and Ionic Radii 原子和离子半径


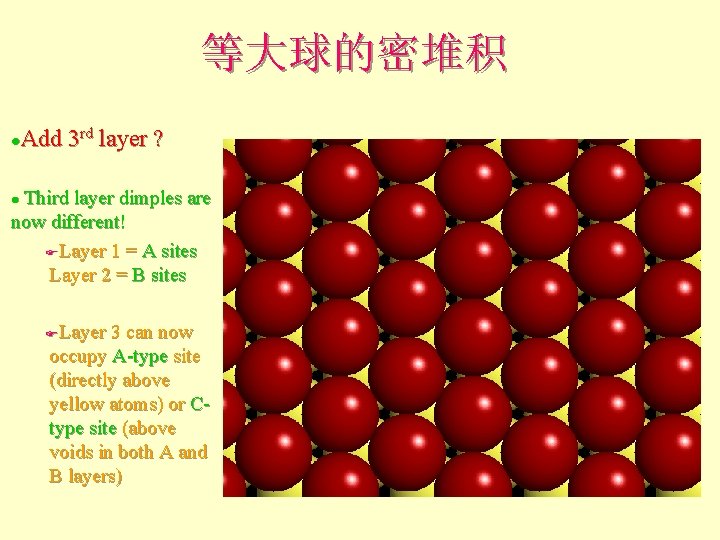
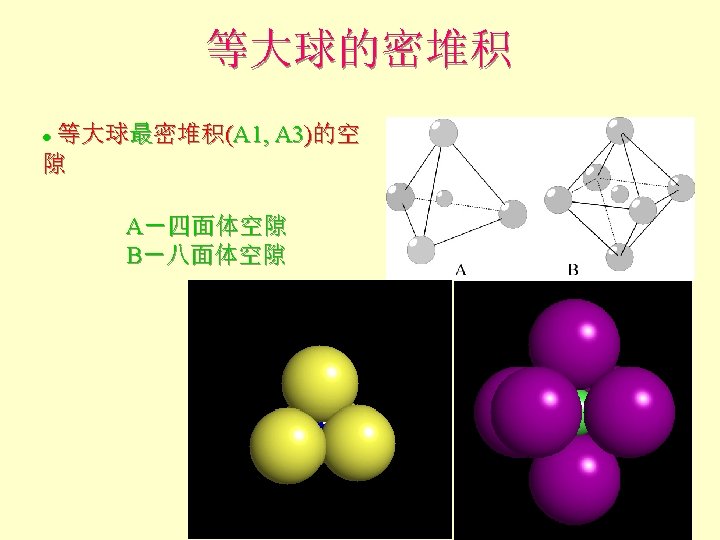
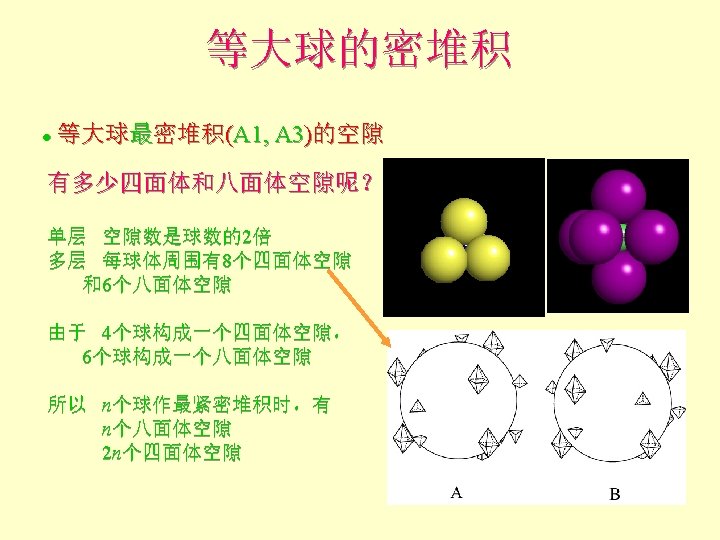
等大球的密堆积 Add 3 rd layer ? l Third layer dimples are now different! FLayer 1 = A sites Layer 2 = B sites l Layer 3 can now occupy A-type site (directly above yellow atoms) or Ctype site (above voids in both A and B layers) F
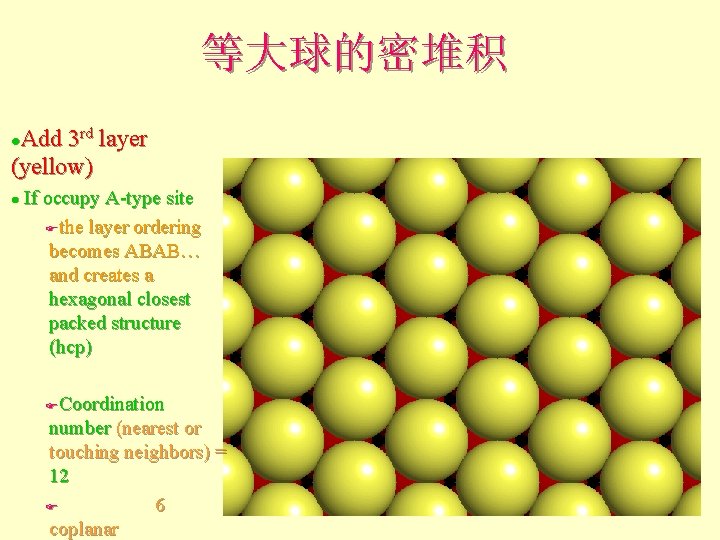
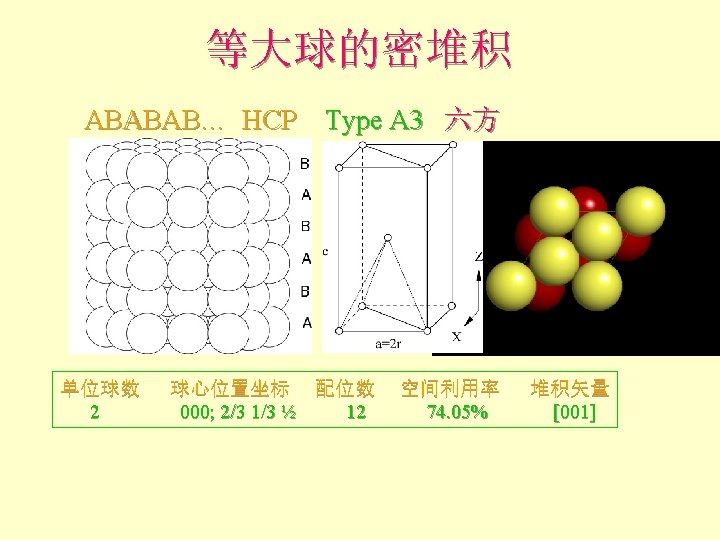
等大球的密堆积 Add 3 rd layer (yellow) l If occupy A-type site Fthe layer ordering becomes ABAB… and creates a hexagonal closest packed structure (hcp) l Coordination number (nearest or touching neighbors) = 12 F 6 coplanar F
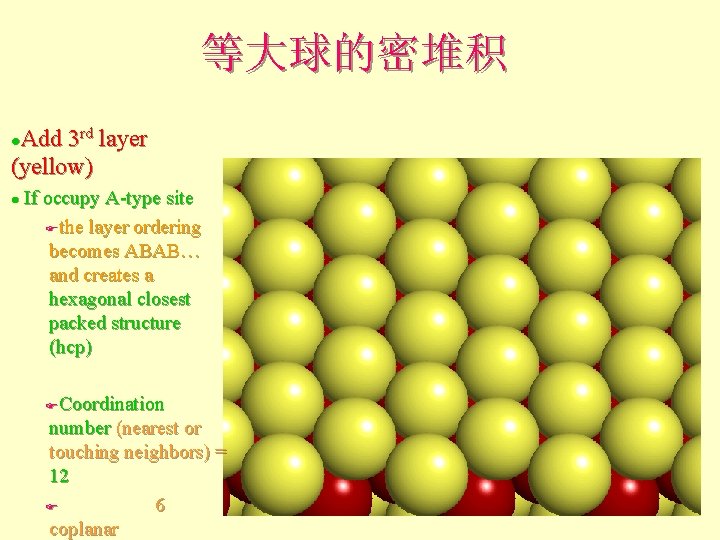
等大球的密堆积 Add 3 rd layer (yellow) l If occupy A-type site Fthe layer ordering becomes ABAB… and creates a hexagonal closest packed structure (hcp) l Coordination number (nearest or touching neighbors) = 12 F 6 coplanar F
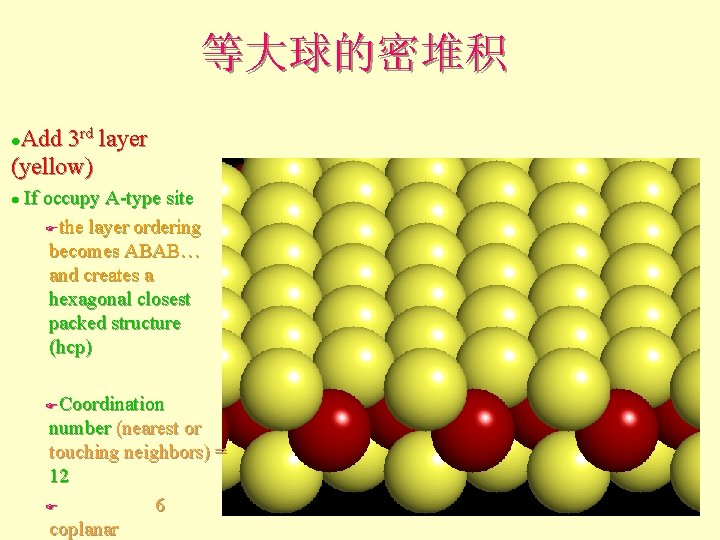
等大球的密堆积 Add 3 rd layer (yellow) l If occupy A-type site Fthe layer ordering becomes ABAB… and creates a hexagonal closest packed structure (hcp) l Coordination number (nearest or touching neighbors) = 12 F 6 coplanar F
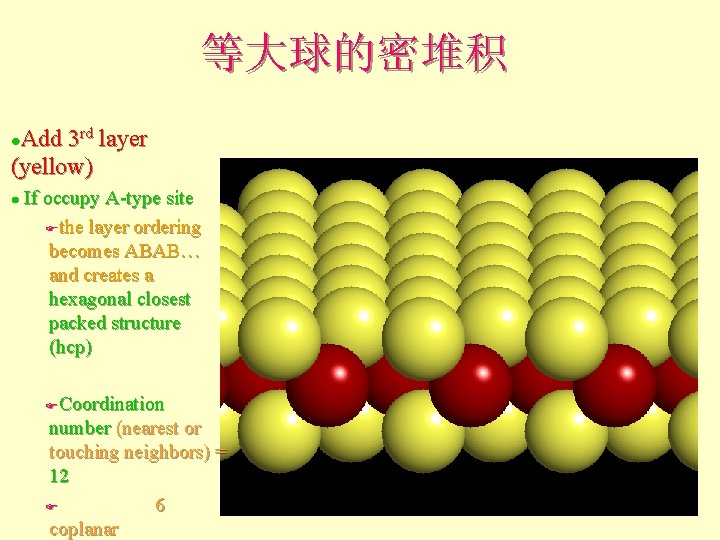
等大球的密堆积 Add 3 rd layer (yellow) l If occupy A-type site Fthe layer ordering becomes ABAB… and creates a hexagonal closest packed structure (hcp) l Coordination number (nearest or touching neighbors) = 12 F 6 coplanar F
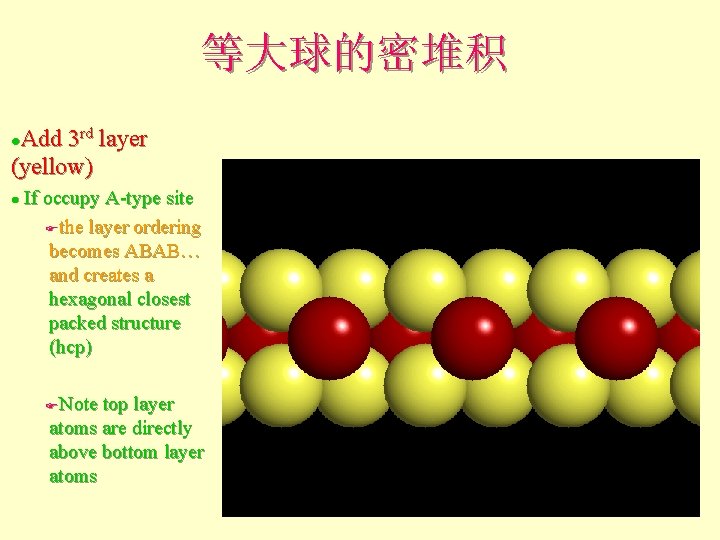
等大球的密堆积 Add 3 rd layer (yellow) l If occupy A-type site Fthe layer ordering becomes ABAB… and creates a hexagonal closest packed structure (hcp) l Note top layer atoms are directly above bottom layer atoms F
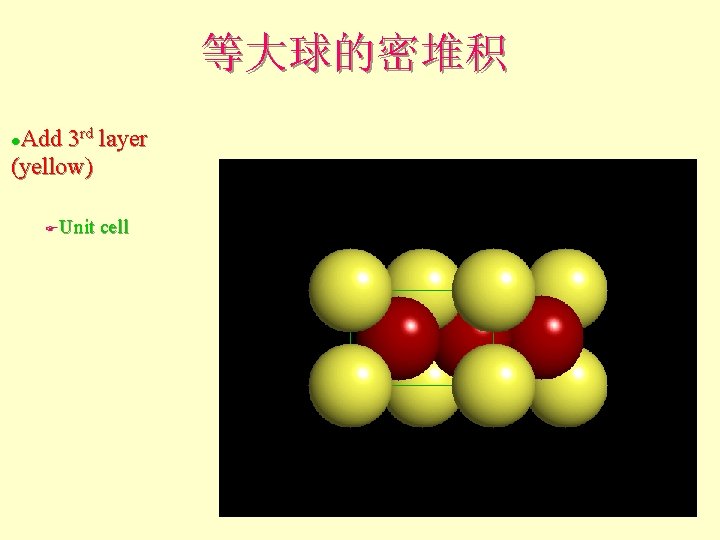
等大球的密堆积 Add 3 rd layer (yellow) l Unit cell F
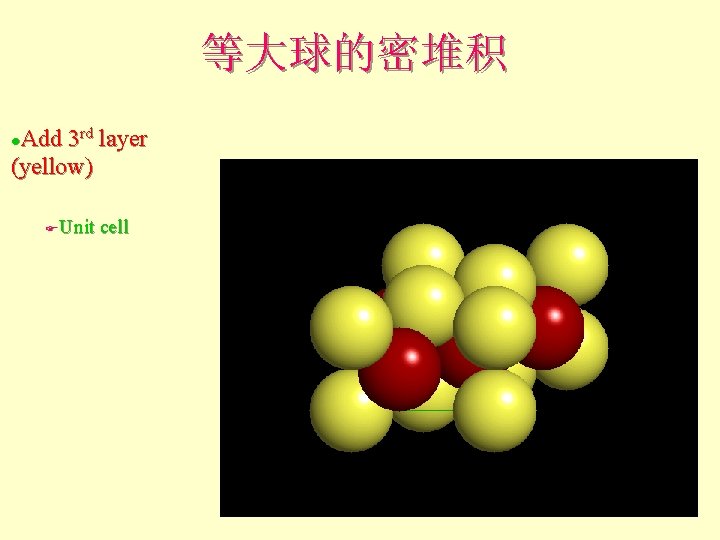
等大球的密堆积 Add 3 rd layer (yellow) l Unit cell F
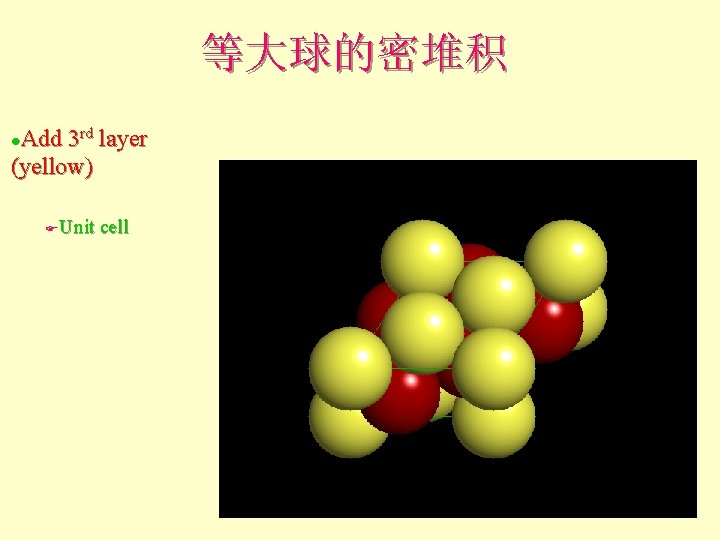
等大球的密堆积 Add 3 rd layer (yellow) l Unit cell F
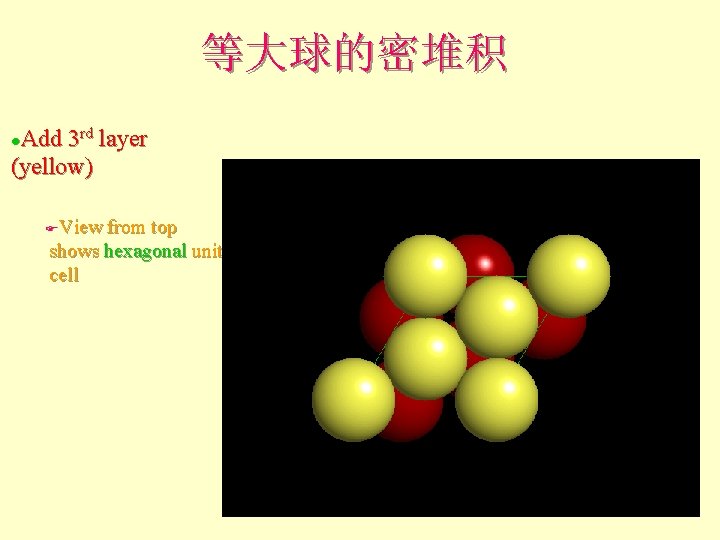
等大球的密堆积 Add 3 rd layer (yellow) l View from top shows hexagonal unit cell F
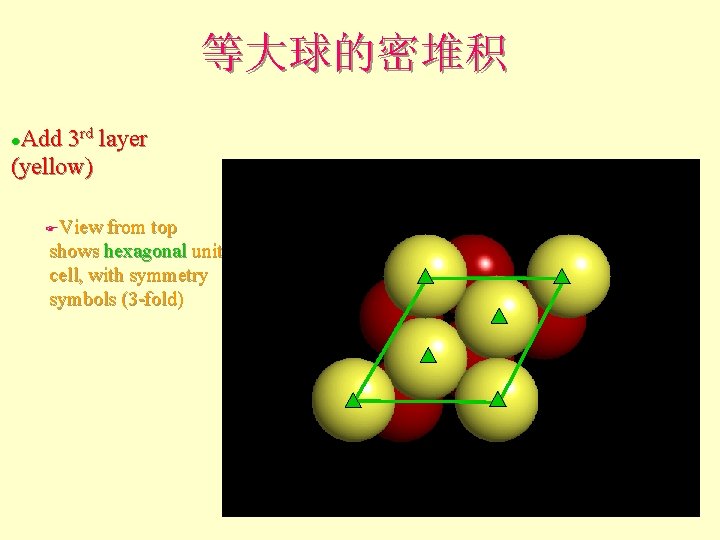
等大球的密堆积 Add 3 rd layer (yellow) l View from top shows hexagonal unit cell, with symmetry symbols (3 -fold) F
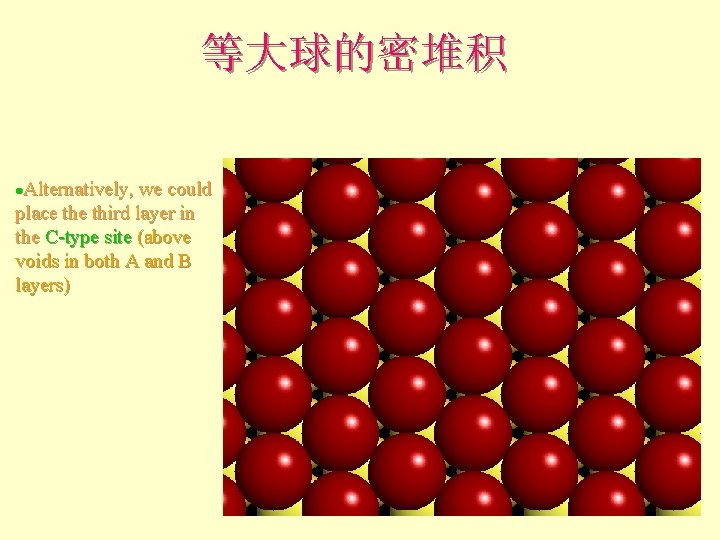
等大球的密堆积 Alternatively, we could place third layer in the C-type site (above voids in both A and B layers) l
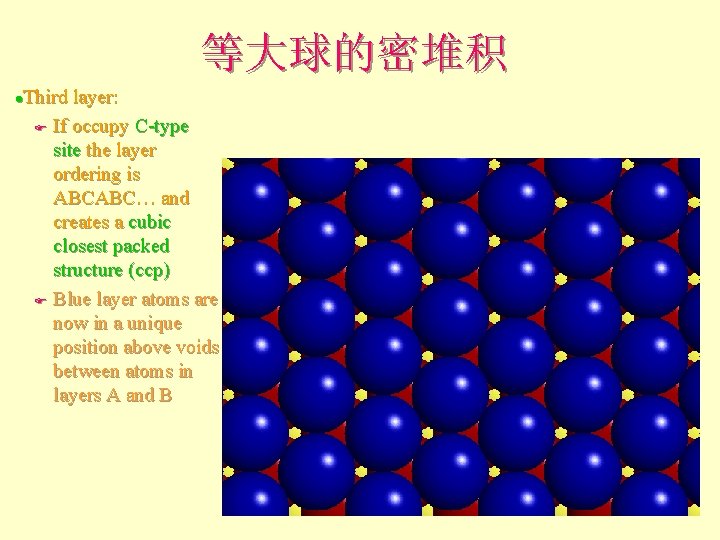
等大球的密堆积 Third layer: F If occupy C-type site the layer ordering is ABCABC… and creates a cubic closest packed structure (ccp) F Blue layer atoms are now in a unique position above voids between atoms in layers A and B l
等大球的密堆积 Third layer: F If occupy C-type site the layer ordering is ABCABC… and creates a cubic closest packed structure (CCP) F Blue layer atoms are now in a unique position above voids between atoms in layers A and B l
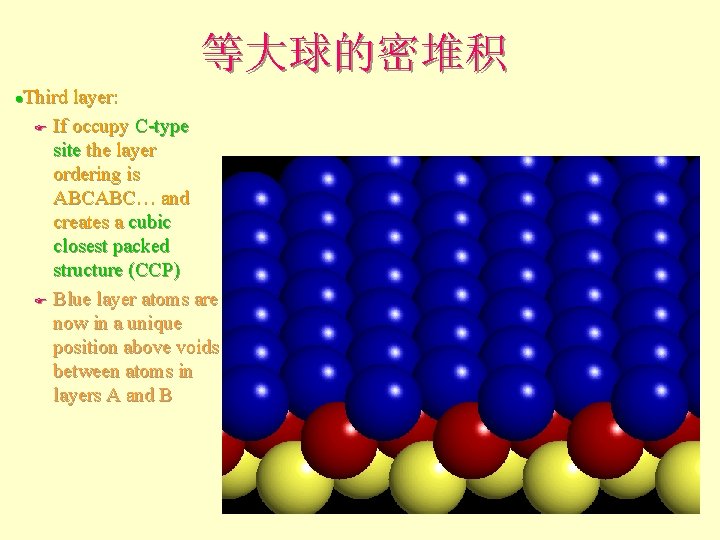
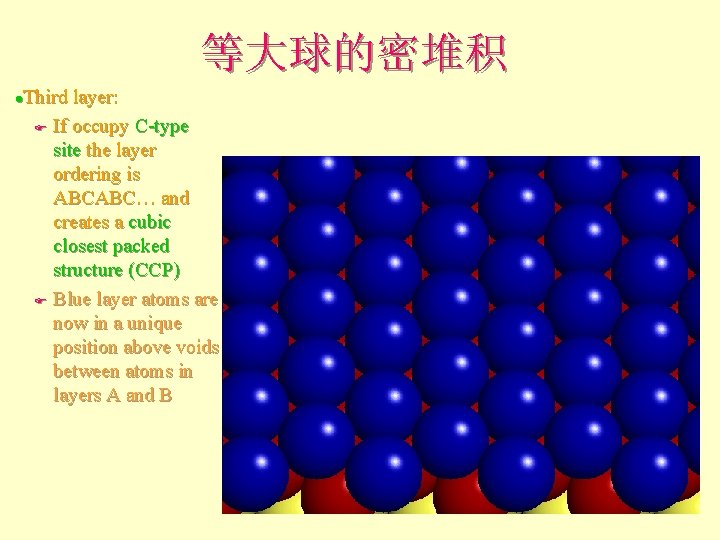
等大球的密堆积 Third layer: F If occupy C-type site the layer ordering is ABCABC… and creates a cubic closest packed structure (CCP) F Blue layer atoms are now in a unique position above voids between atoms in layers A and B l
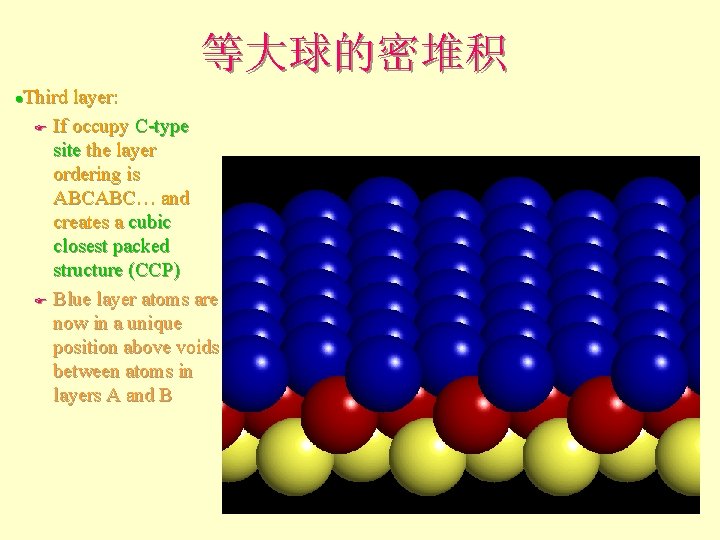
等大球的密堆积 Third layer: F If occupy C-type site the layer ordering is ABCABC… and creates a cubic closest packed structure (CCP) F Blue layer atoms are now in a unique position above voids between atoms in layers A and B l
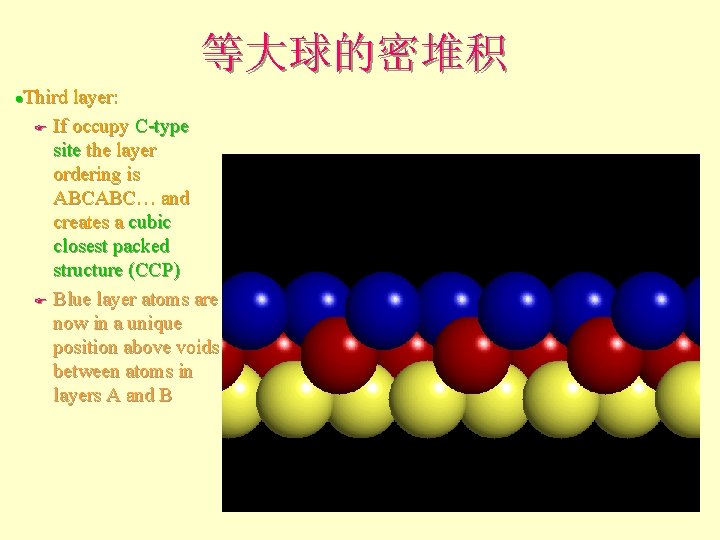
等大球的密堆积 Third layer: F If occupy C-type site the layer ordering is ABCABC… and creates a cubic closest packed structure (CCP) F Blue layer atoms are now in a unique position above voids between atoms in layers A and B l
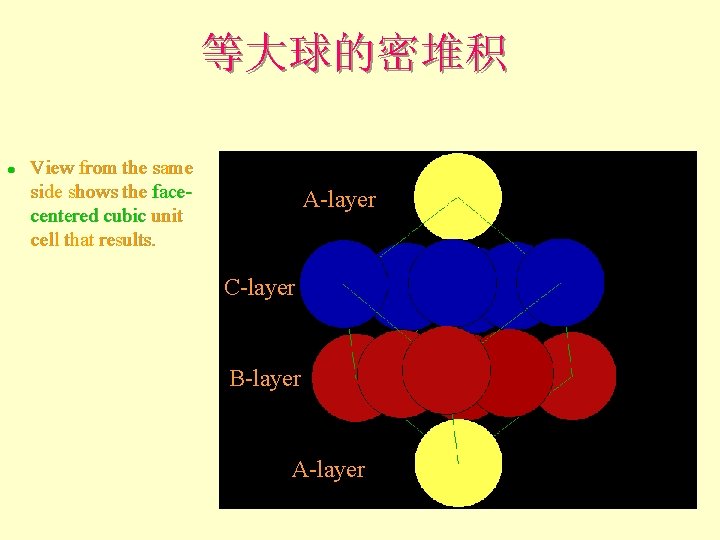
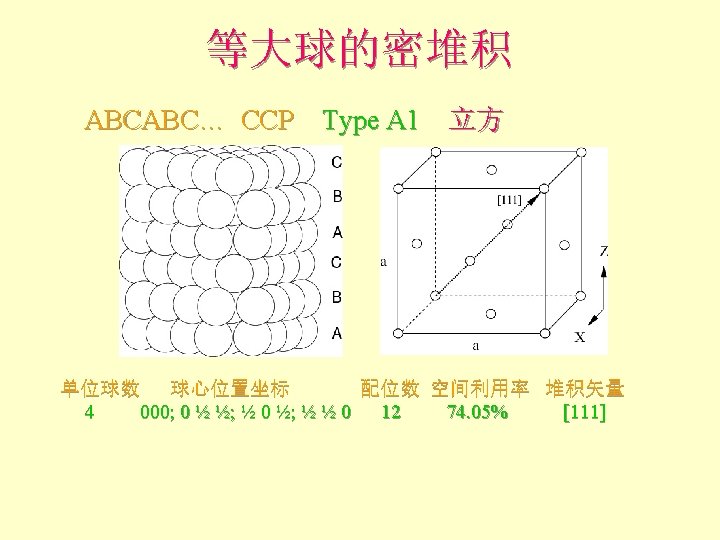
等大球的密堆积 l View from the same side shows the facecentered cubic unit cell that results. A-layer C-layer B-layer A-layer
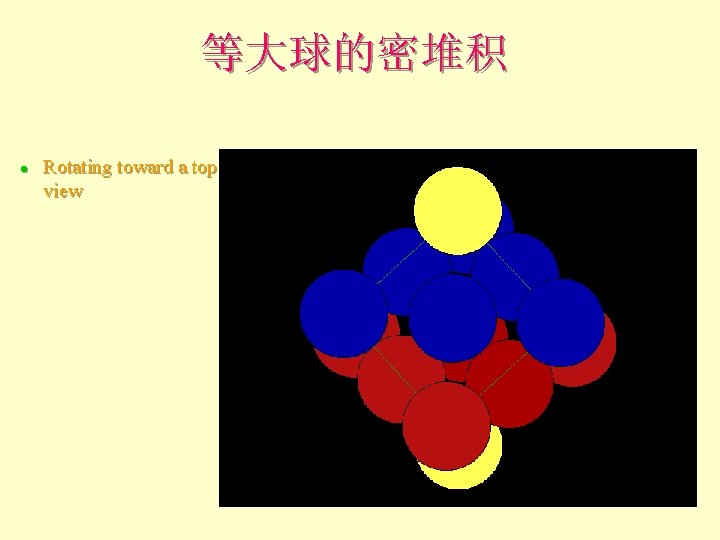
等大球的密堆积 l Rotating toward a top view
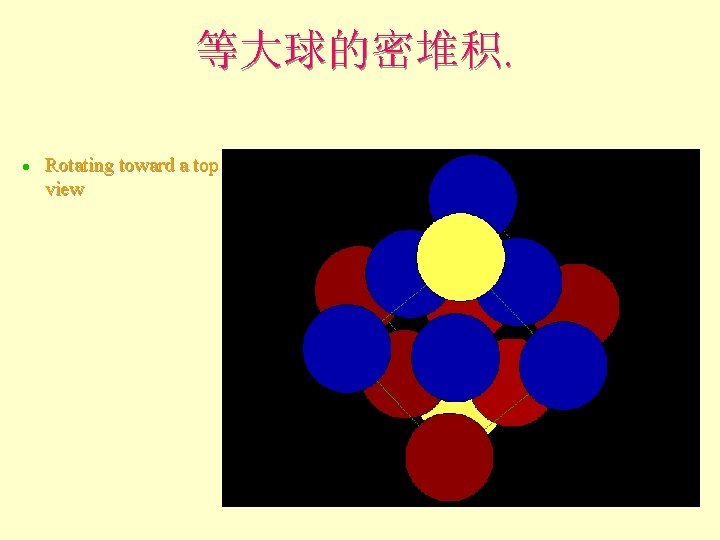
等大球的密堆积. l Rotating toward a top view
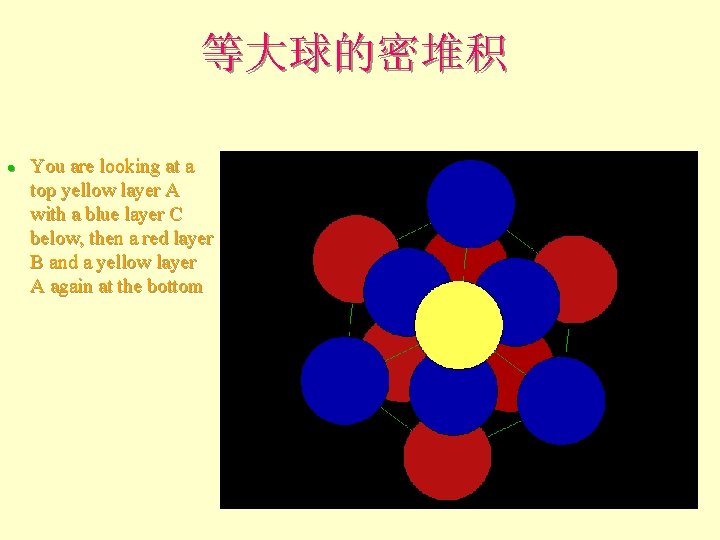
等大球的密堆积 l You are looking at a top yellow layer A with a blue layer C below, then a red layer B and a yellow layer A again at the bottom
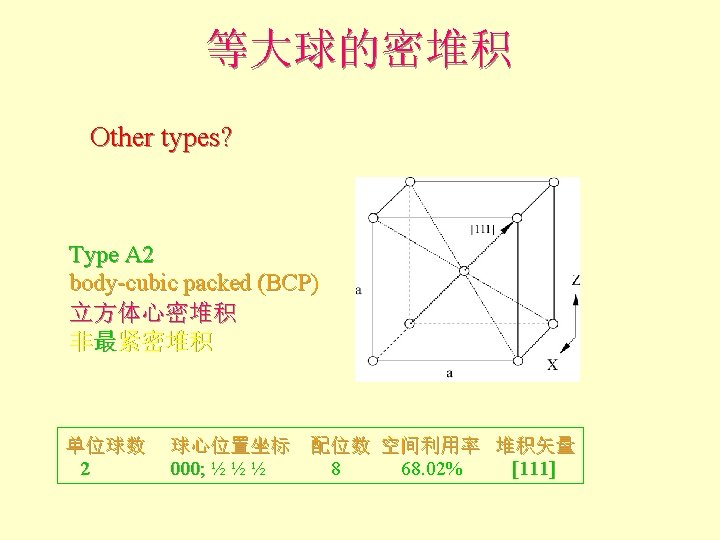
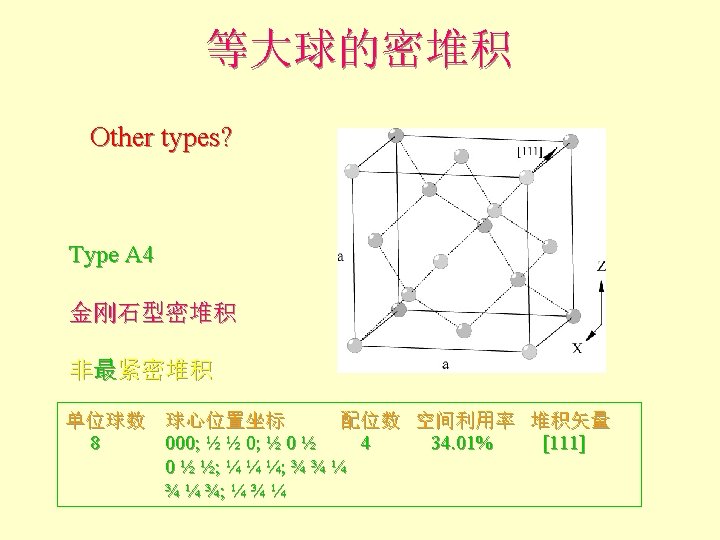
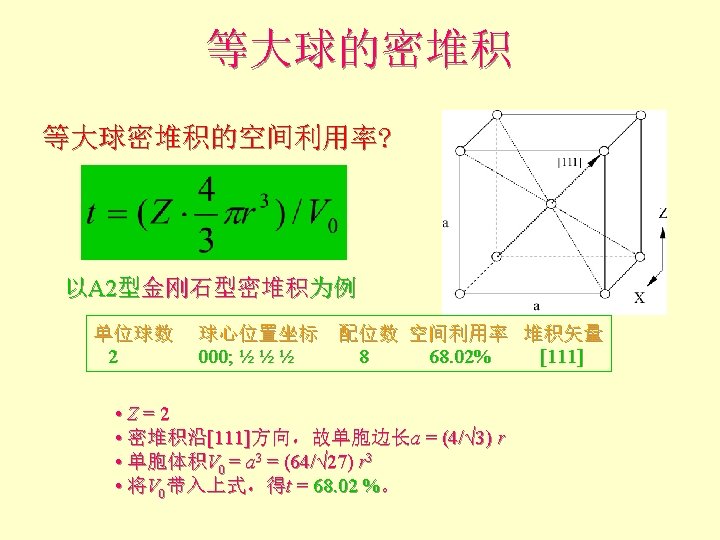

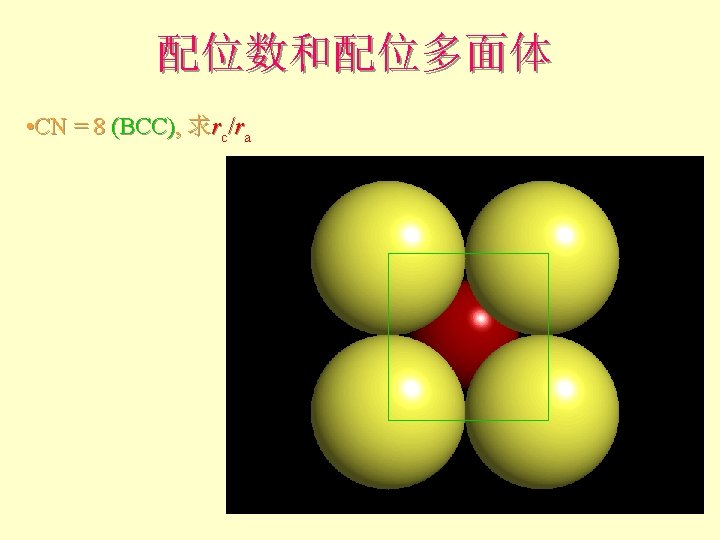
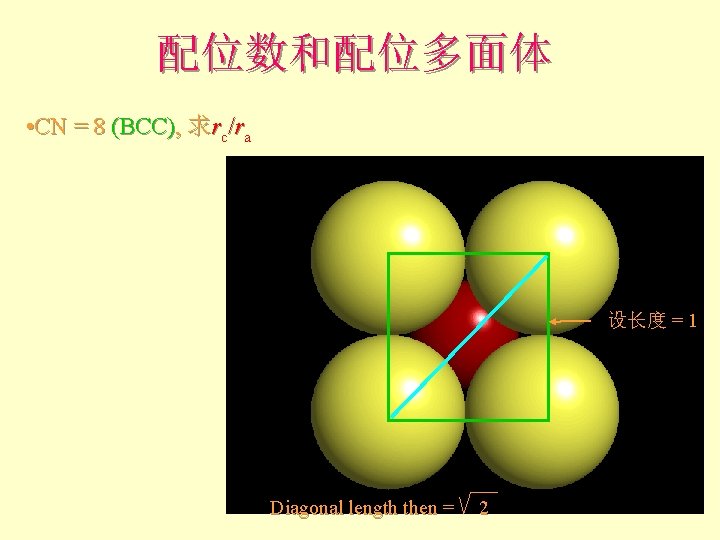
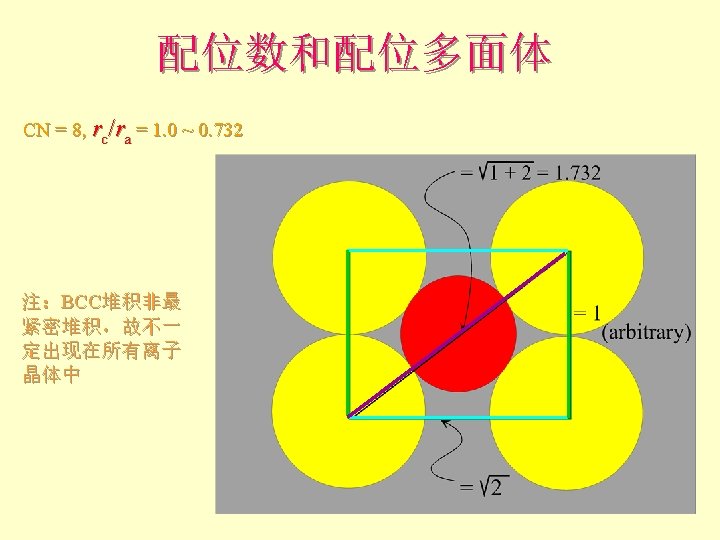
配位数和配位多面体 • CN = 8 (BCC), 求rc/ra 设长度 = 1 Diagonal length then = 2
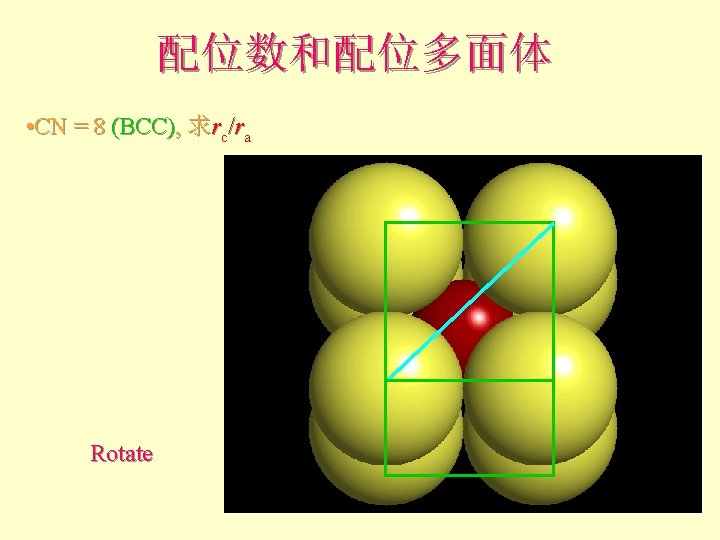
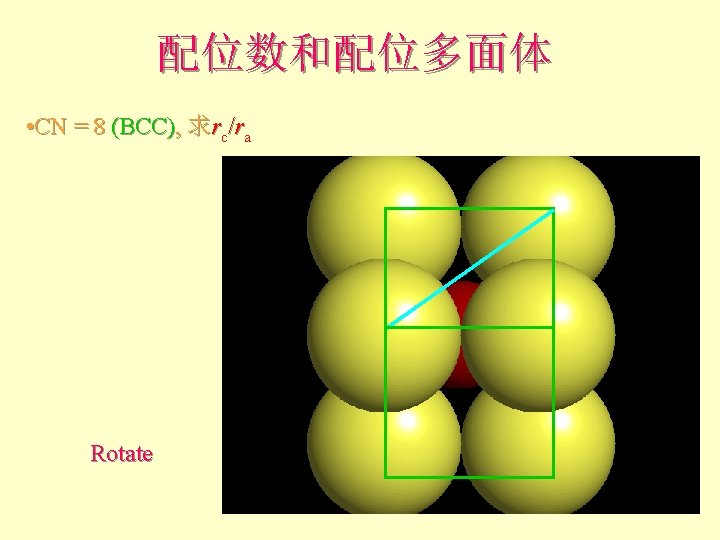
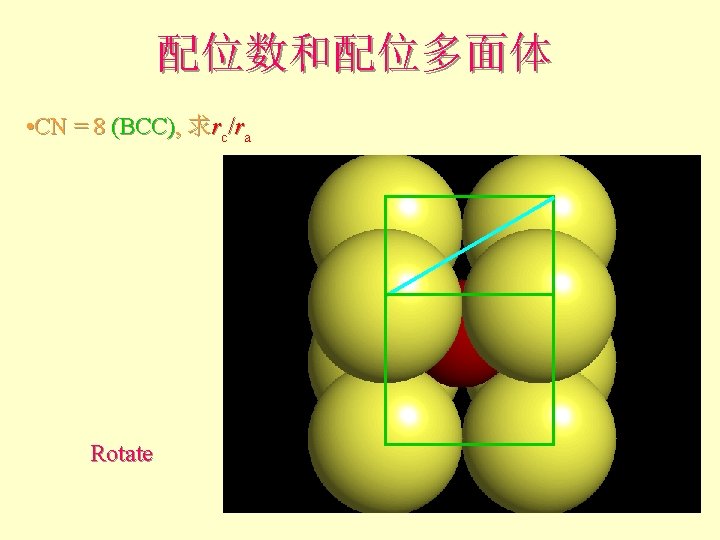
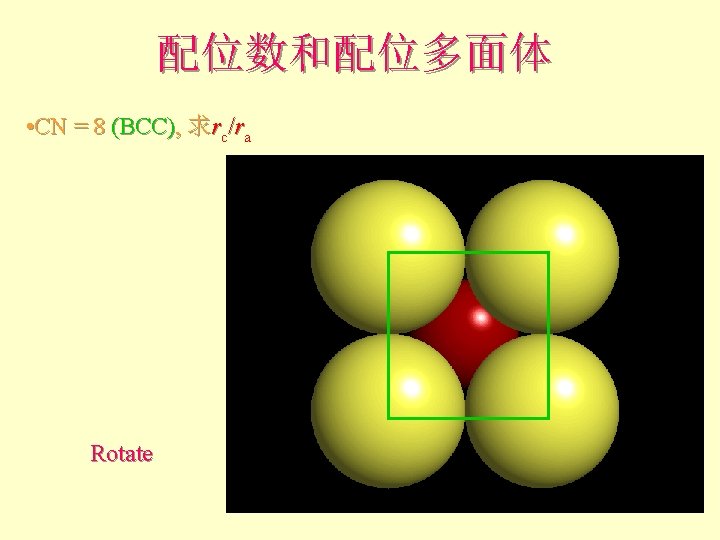
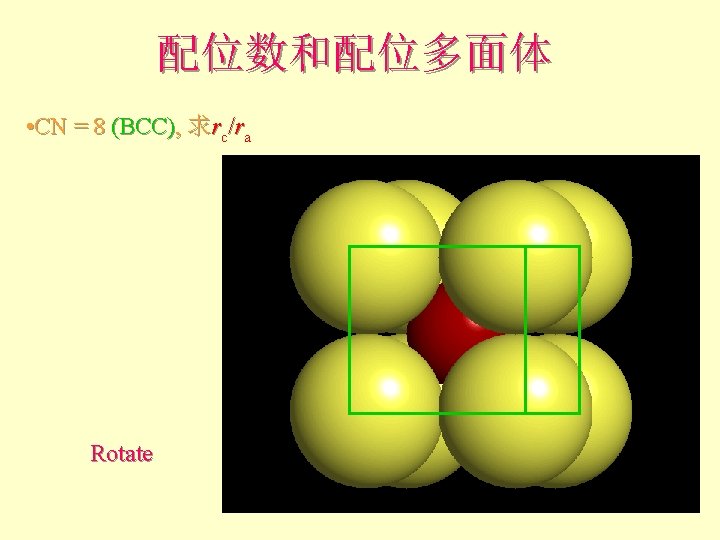
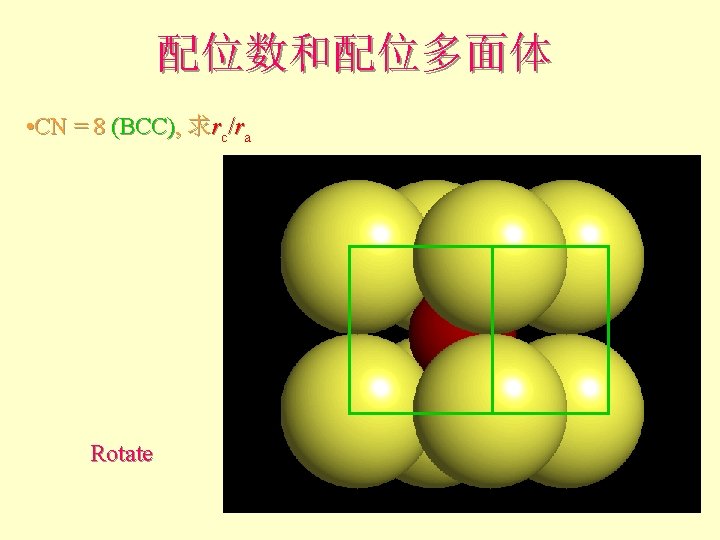
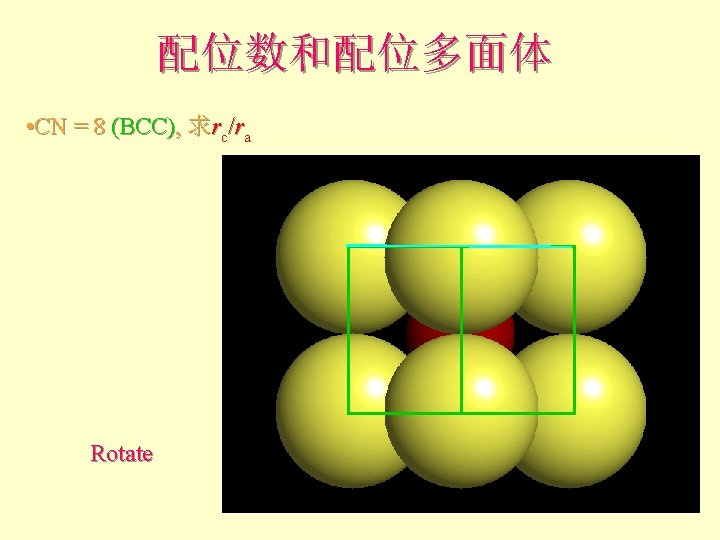
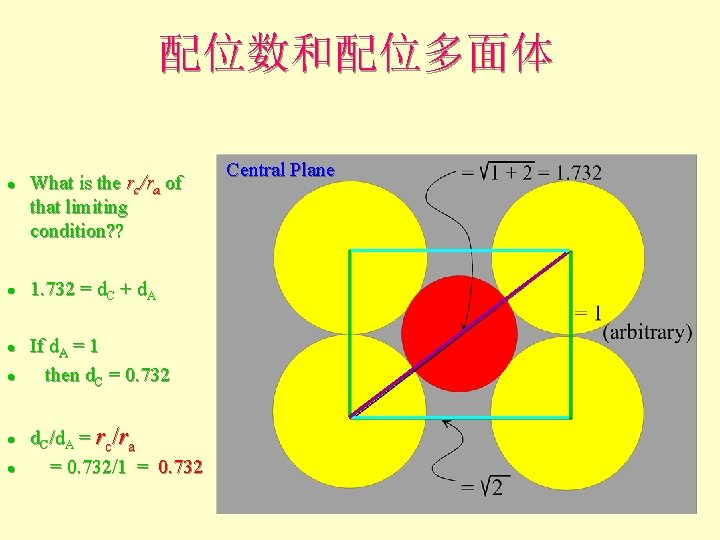
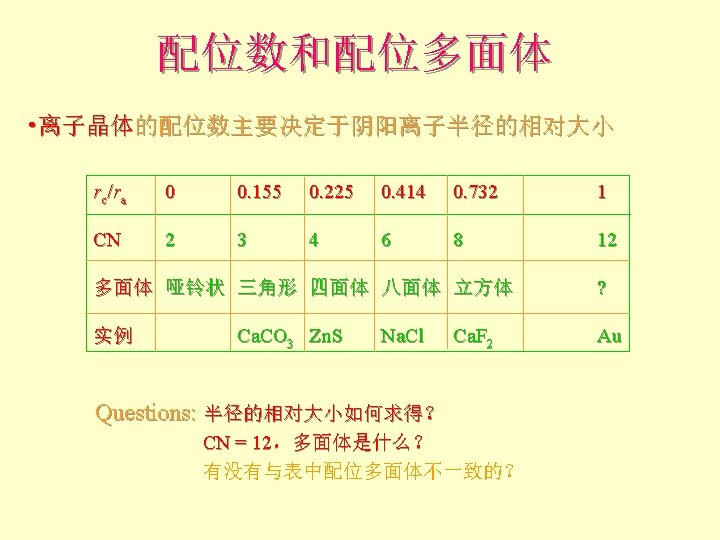
配位数和配位多面体 l l l What is the rc/ra of that limiting condition? ? 1. 732 = d. C + d. A If d. A = 1 then d. C = 0. 732 d. C/d. A = rc/ra = 0. 732/1 = 0. 732 Central Plane
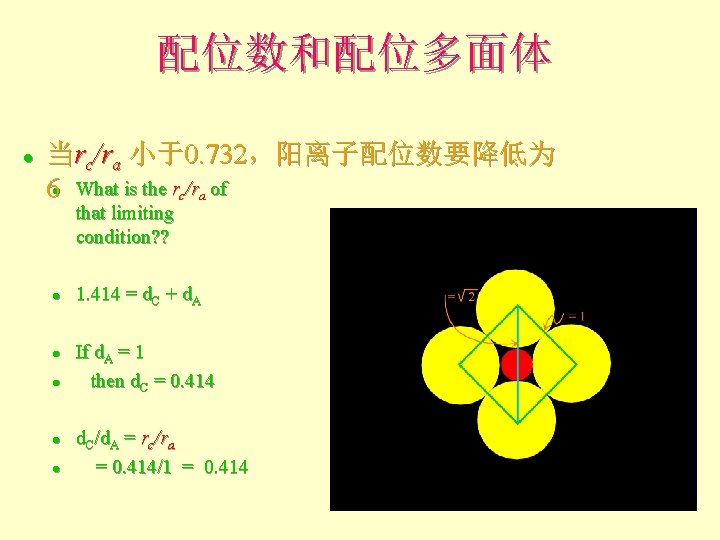
配位数和配位多面体 l 当rc/ra 小于0. 732,阳离子配位数要降低为 6 What is the rc/ra of l that limiting condition? ? l l l 1. 414 = d. C + d. A If d. A = 1 then d. C = 0. 414 d. C/d. A = rc/ra = 0. 414/1 = 0. 414

配位数和配位多面体 l l 当rc/ra 小于0. 414,阳离子配位数要降低为 4 What is the rc/ra of the limiting condition? ? l 中心到角顶的距离 = 0. 6124 l rc = 0. 612 - 0. 5 = 0. 1124 l l rc / ra = 0. 1124/0. 5 = 0. 225
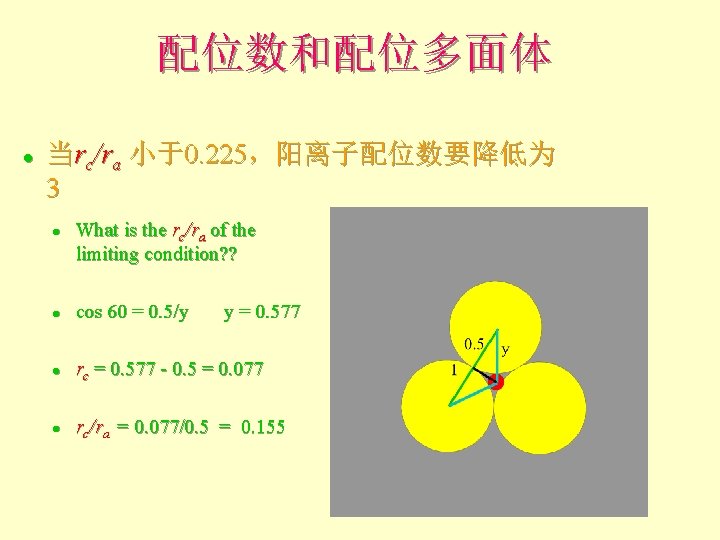
配位数和配位多面体 l 当rc/ra 小于0. 225,阳离子配位数要降低为 3 l What is the rc/ra of the limiting condition? ? l cos 60 = 0. 5/y y = 0. 577 l rc = 0. 577 - 0. 5 = 0. 077 l rc/ra = 0. 077/0. 5 = 0. 155
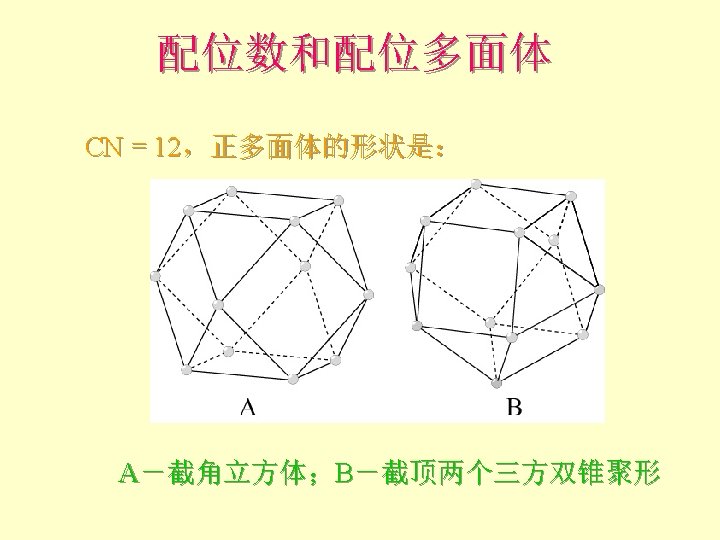



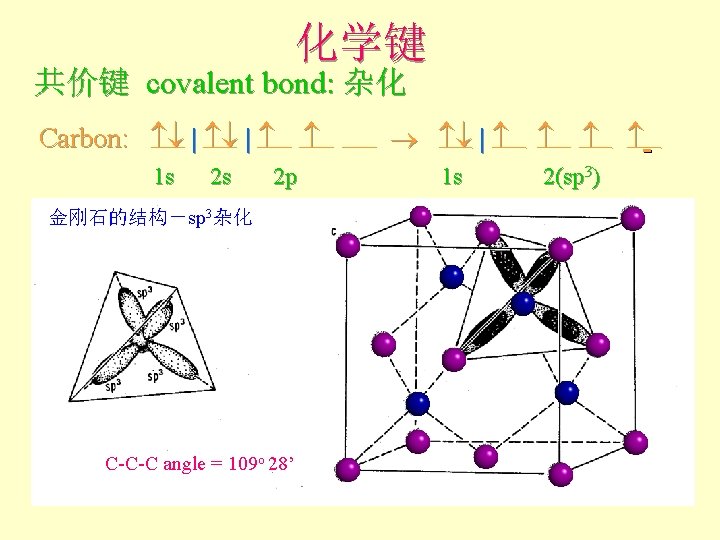
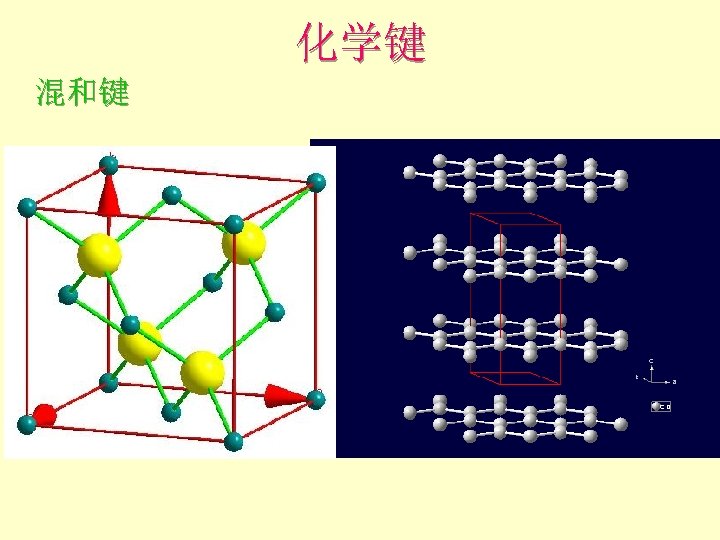
化学键 共价键 covalent bond: 杂化 Carbon: | | 1 s 2 s 2 p 金刚石的结构-sp 3杂化 C-C-C angle = 109 o 28’ 1 s 2(sp 3)
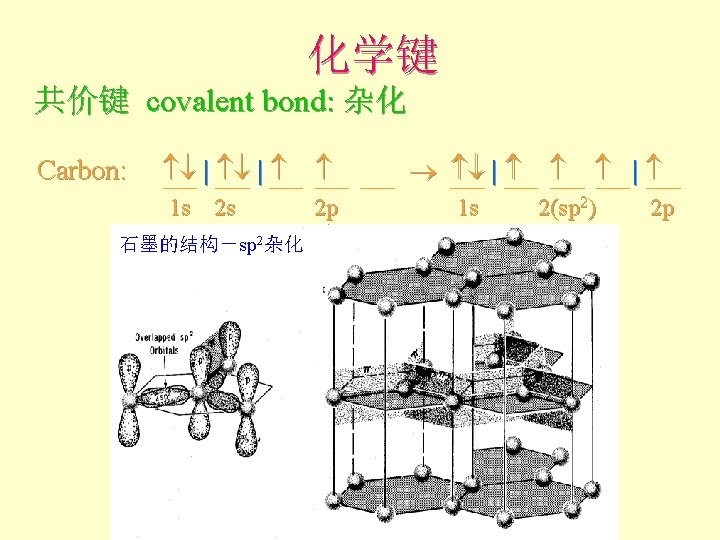
化学键 共价键 covalent bond: 杂化 Carbon: | | 1 s 2 s 2 p 石墨的结构-sp 2杂化 1 s 2(sp 2) 2 p

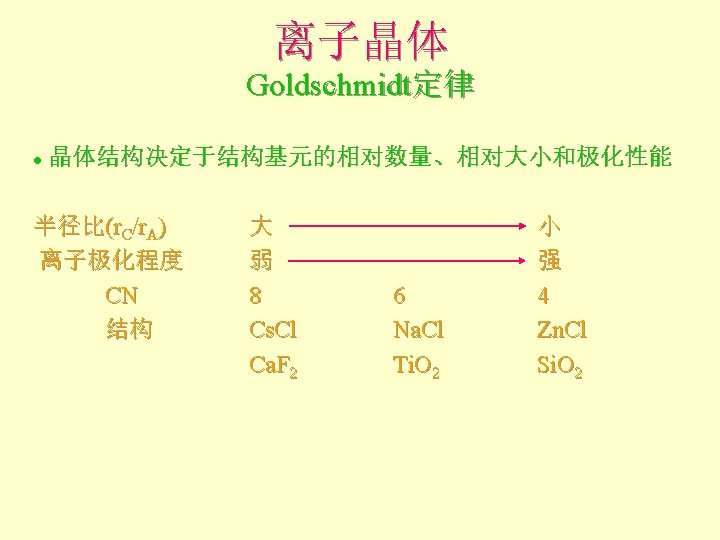

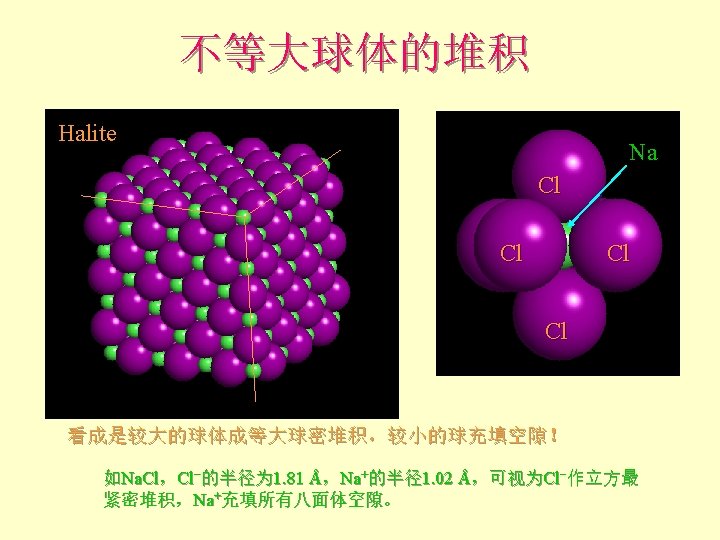
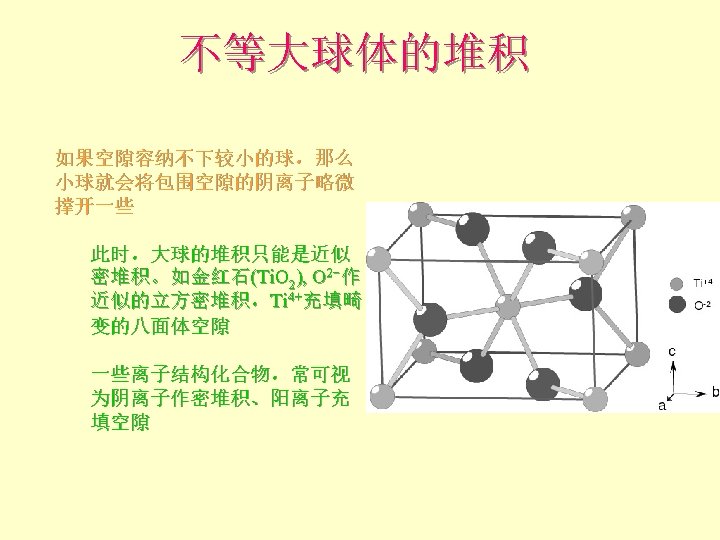
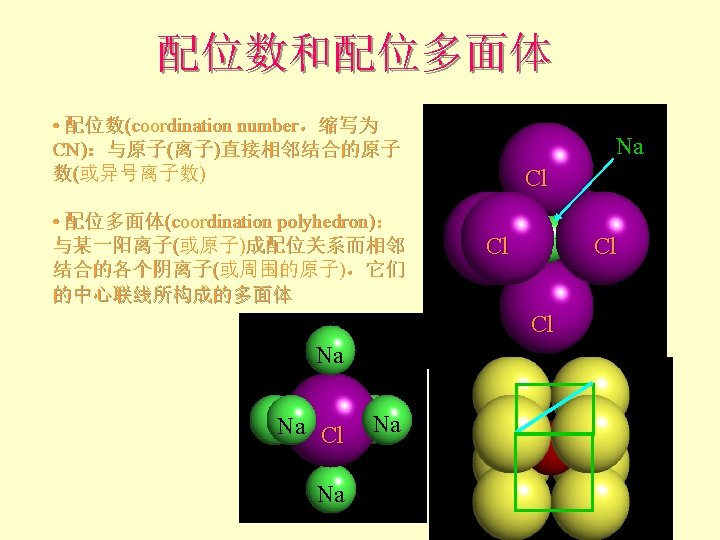
离子晶体 Pauling规则 1 st Rule l The cation-anion distance = radii Can use RC/RA to determine the coordination number of the cation 半径规则:围绕阳离子形成一个阴离子配位多面体, 阴阳离子间距取决于它们的半径和,配位数取决于其 半径比。
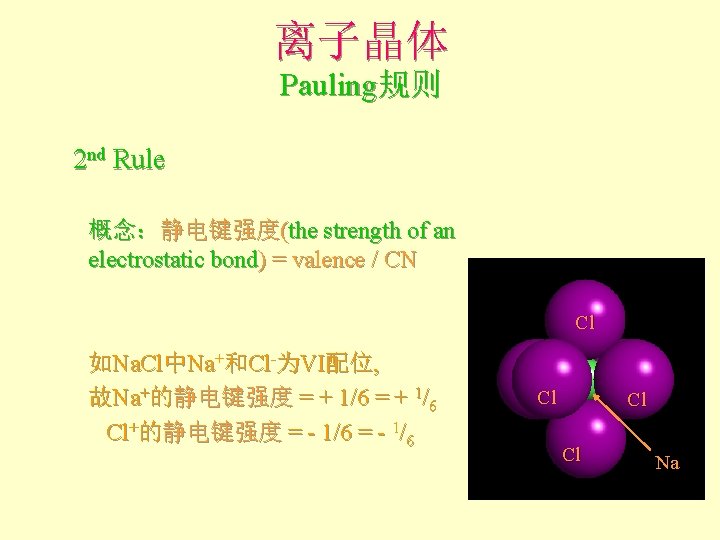
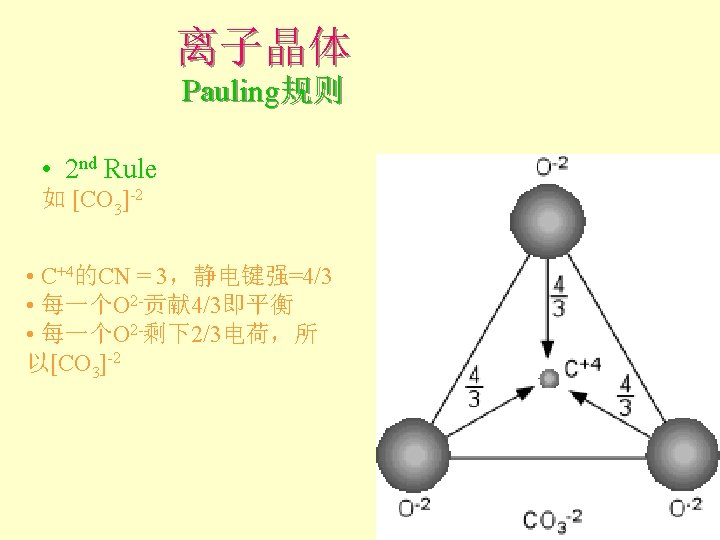
离子晶体 Pauling规则 2 nd Rule 概念:静电键强度(the strength of an electrostatic bond) = valence / CN Cl 如Na. Cl中Na+和Cl-为VI配位, 故Na+的静电键强度 = + 1/6 Cl+的静电键强度 = - 1/6 Cl Cl Cl Na

离子晶体 Pauling规则 • 2 nd Rule: the electrostatic valence principle An ionic structure will be stable to the extent that the sum of the strengths of the electrostatic bonds that reach an ion equal the charge on that ion. l 电价规则:稳定离子结构的离子电价等于 与其相邻异号离子的各静电键强度的总和
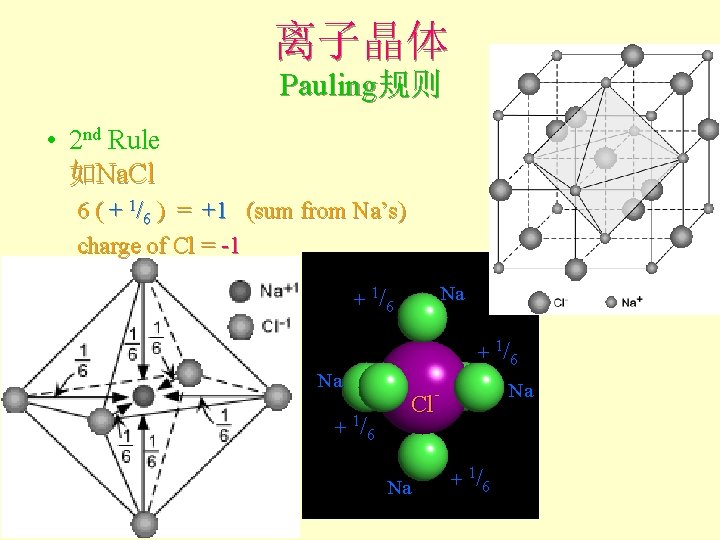
离子晶体 Pauling规则 • 2 nd Rule 如Na. Cl 6 ( + 1/6 ) = +1 (sum from Na’s) charge of Cl = -1 Na + 1/6 Na - Cl. Na + 1/6
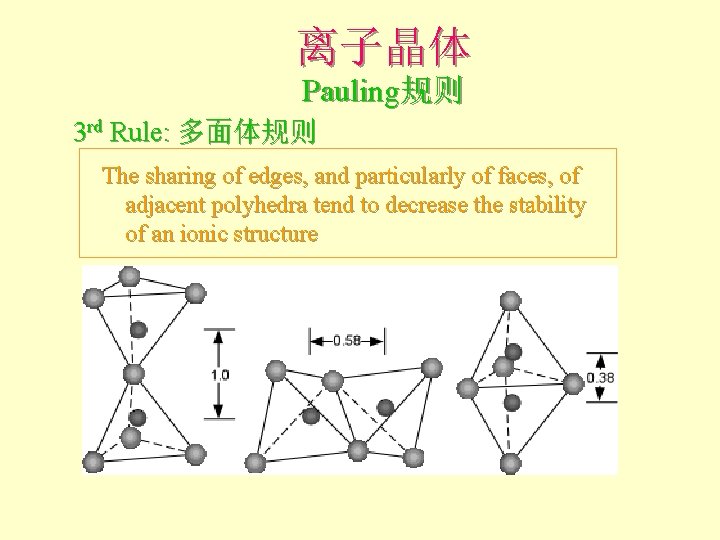
离子晶体 Pauling规则 3 rd Rule: 多面体规则 The sharing of edges, and particularly of faces, of adjacent polyhedra tend to decrease the stability of an ionic structure
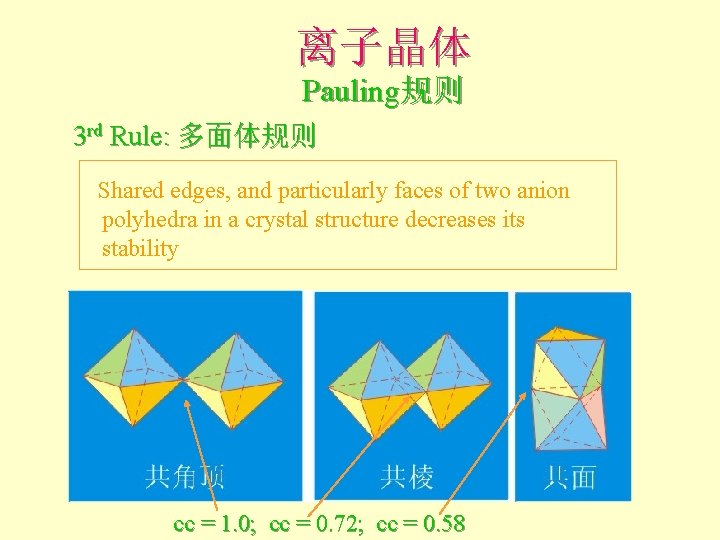
离子晶体 Pauling规则 3 rd Rule: 多面体规则 Shared edges, and particularly faces of two anion polyhedra in a crystal structure decreases its stability cc = 1. 0; cc = 0. 72; cc = 0. 58
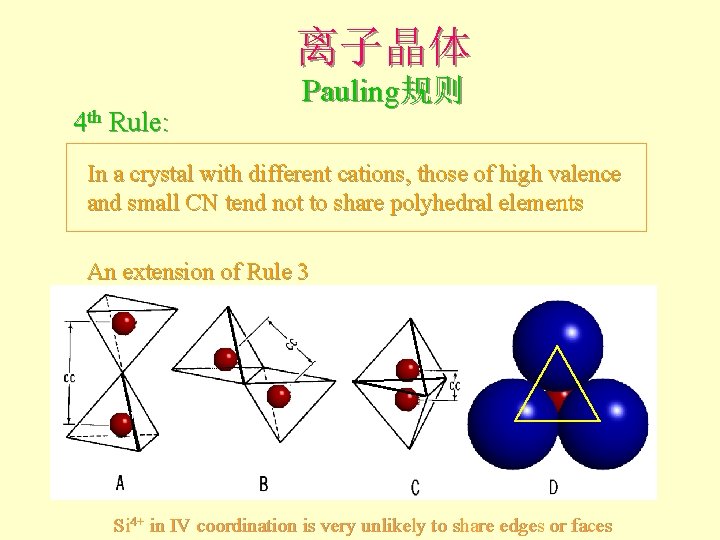
离子晶体 4 th Rule: Pauling规则 In a crystal with different cations, those of high valence and small CN tend not to share polyhedral elements An extension of Rule 3 Si 4+ in IV coordination is very unlikely to share edges or faces

离子晶体 Pauling规则 5 th Rule: 最简规则 The number of different kinds of constituents in a crystal tends to be small. This means that there are only a few different types of cation and anion sites in a crystal. Even though a crystal may have tetrahedral sites, octahedral sites, and cubic sites, most crystals will be limited to this small number of sites, although different elements may occupy similar sites.
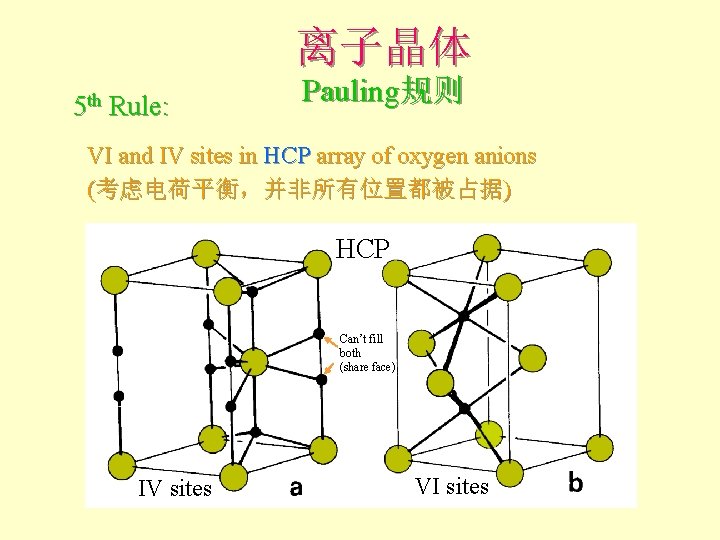
离子晶体 5 th Rule: Pauling规则 VI and IV sites in HCP array of oxygen anions (考虑电荷平衡,并非所有位置都被占据) HCP Can’t fill both (share face) IV sites VI sites
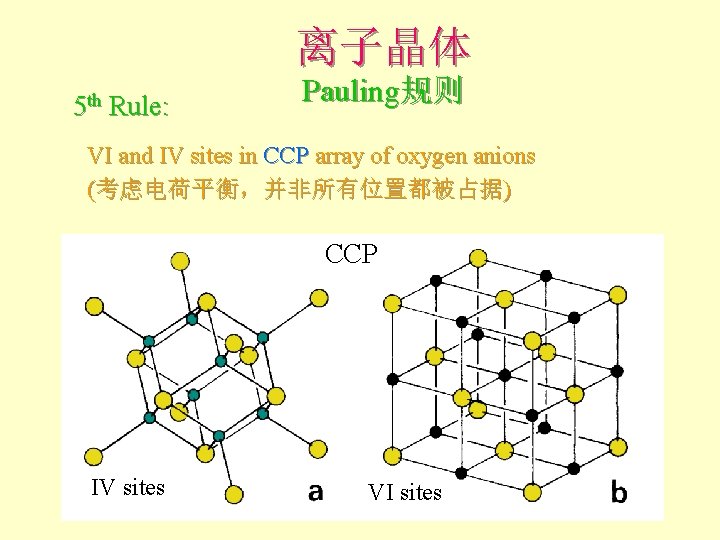
离子晶体 5 th Rule: Pauling规则 VI and IV sites in CCP array of oxygen anions (考虑电荷平衡,并非所有位置都被占据) CCP IV sites VI sites

离子晶体 5 th Rule: Pauling规则 尖晶石结构 (A 2+)IV(B 3+)2 VIO 4 Note CCP O 2 -: ABCABC… 白球:VI sites 蓝球:IV sites
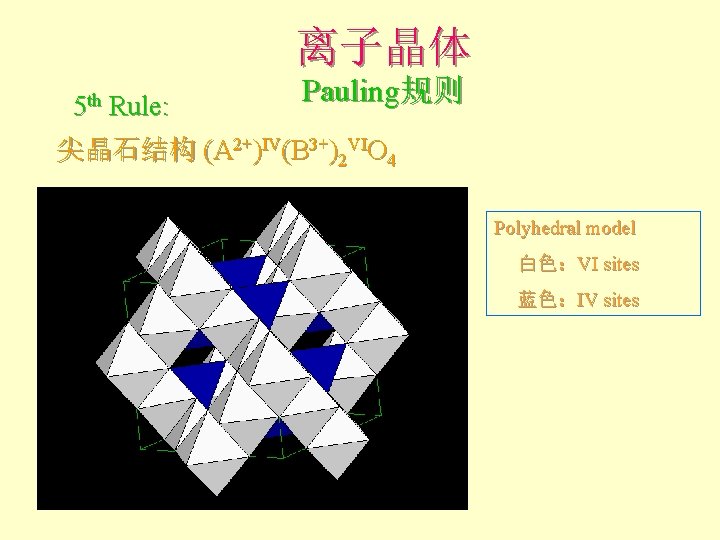
离子晶体 5 th Rule: Pauling规则 尖晶石结构 (A 2+)IV(B 3+)2 VIO 4 Polyhedral model 白色:VI sites 蓝色:IV sites
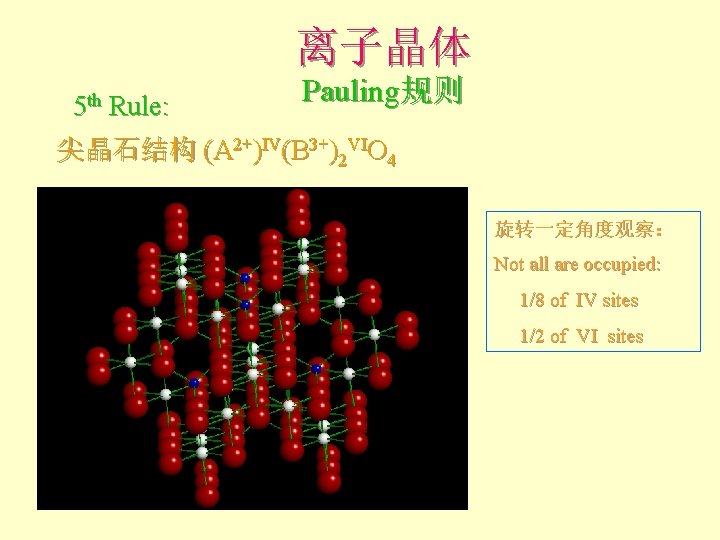
离子晶体 5 th Rule: Pauling规则 尖晶石结构 (A 2+)IV(B 3+)2 VIO 4 旋转一定角度观察: Not all are occupied: 1/8 of IV sites 1/2 of VI sites
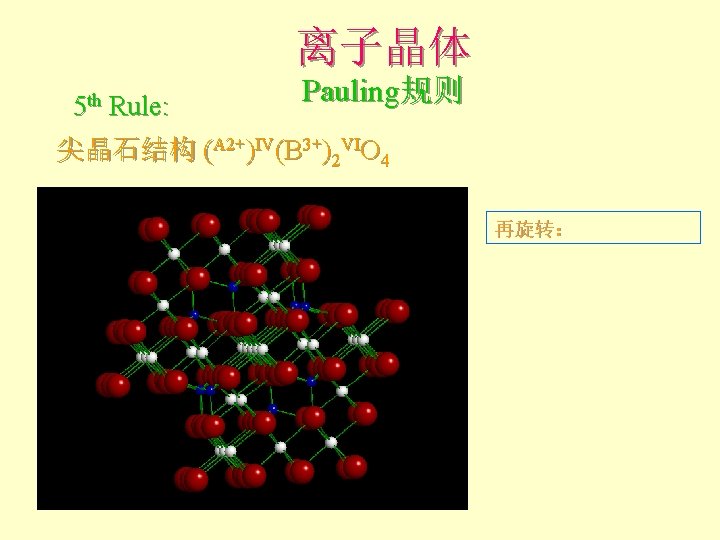
![离子晶体 5 th Rule: Pauling规则 尖晶石结构 (A 2+)IV(B 3+)2 VIO 4 旋转至沿[010]观察 The order 离子晶体 5 th Rule: Pauling规则 尖晶石结构 (A 2+)IV(B 3+)2 VIO 4 旋转至沿[010]观察 The order](http://slidetodoc.com/presentation_image_h/32397ed4dfa3f1f274fe2f69b162d79e/image-105.jpg)
离子晶体 5 th Rule: Pauling规则 尖晶石结构 (A 2+)IV(B 3+)2 VIO 4 旋转至沿[010]观察 The order becomes apparent
- Slides: 105