Putting It All Together Massachusetts Integrated Counseling Testing






- Slides: 19

Putting It All Together: Massachusetts Integrated Counseling, Testing and Referral Program (ICTR) Sheila Nelson, MPH, MSW Daniel Church, MPH; Brenda Cole; H. Dawn Fukuda, Sc. M; David Novak, MSW; Clare O’Donoghue Massachusetts Department of Public Health

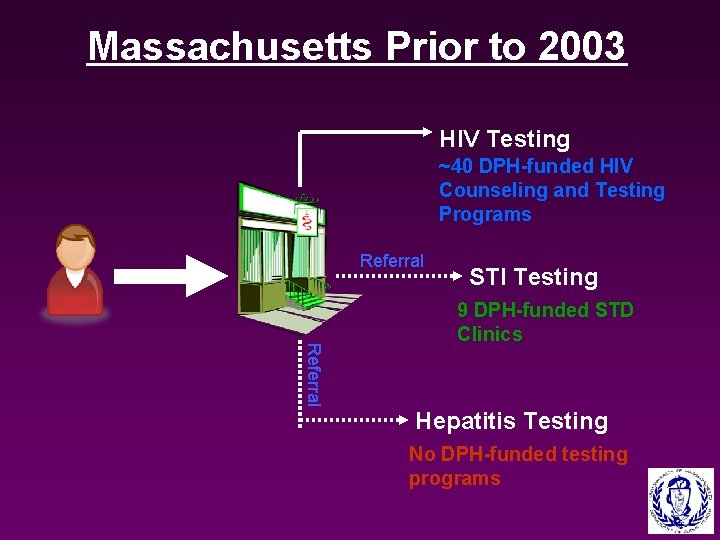
Massachusetts Prior to 2003 HIV Testing ~40 DPH-funded HIV Counseling and Testing Programs Referral STI Testing Referral 9 DPH-funded STD Clinics Hepatitis Testing No DPH-funded testing programs

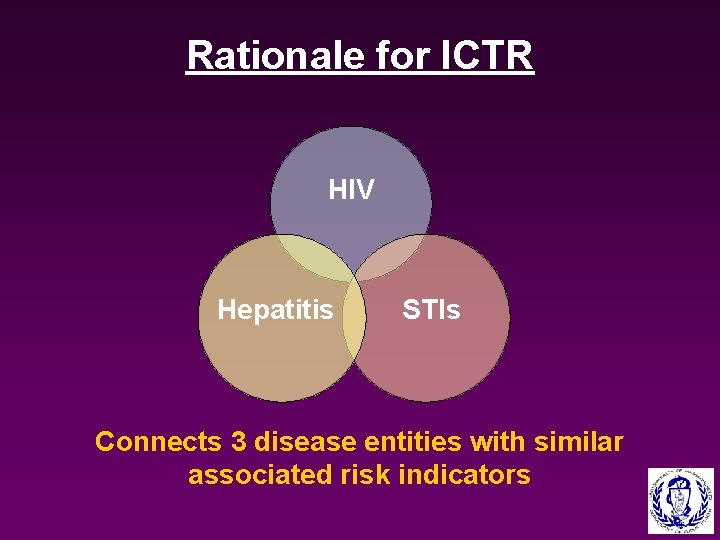
Rationale for ICTR HIV Hepatitis STIs Connects 3 disease entities with similar associated risk indicators

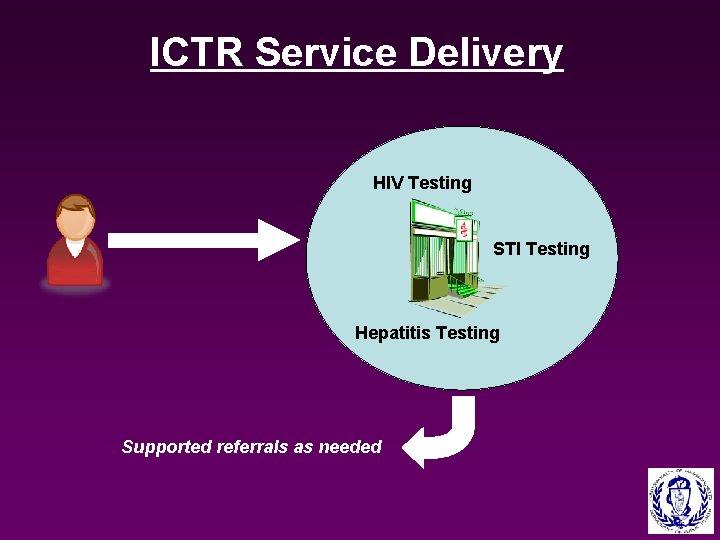
ICTR Service Delivery HIV Testing STI Testing Hepatitis Testing Supported referrals as needed

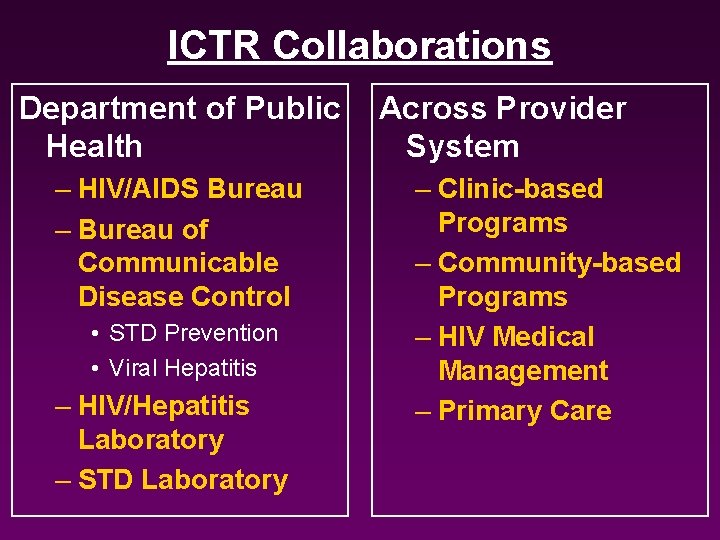
ICTR Collaborations Department of Public Health – HIV/AIDS Bureau – Bureau of Communicable Disease Control • STD Prevention • Viral Hepatitis – HIV/Hepatitis Laboratory – STD Laboratory Across Provider System – Clinic-based Programs – Community-based Programs – HIV Medical Management – Primary Care

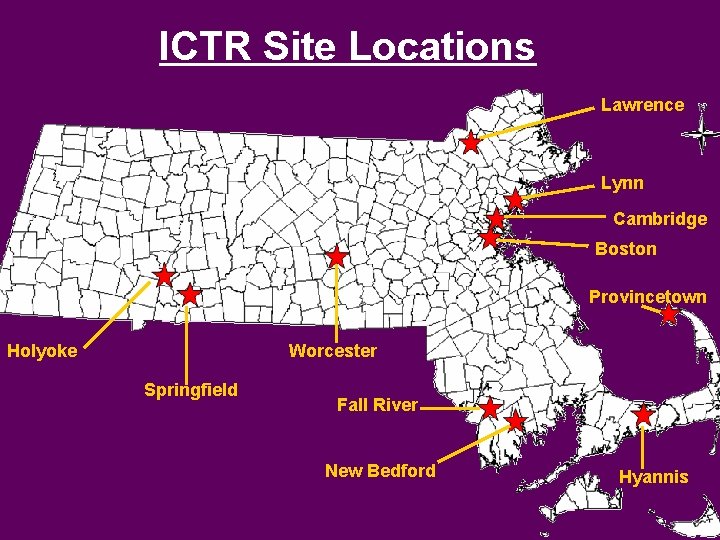
ICTR Site Locations Lawrence Lynn Cambridge Boston Provincetown Holyoke Worcester Springfield Fall River New Bedford Hyannis


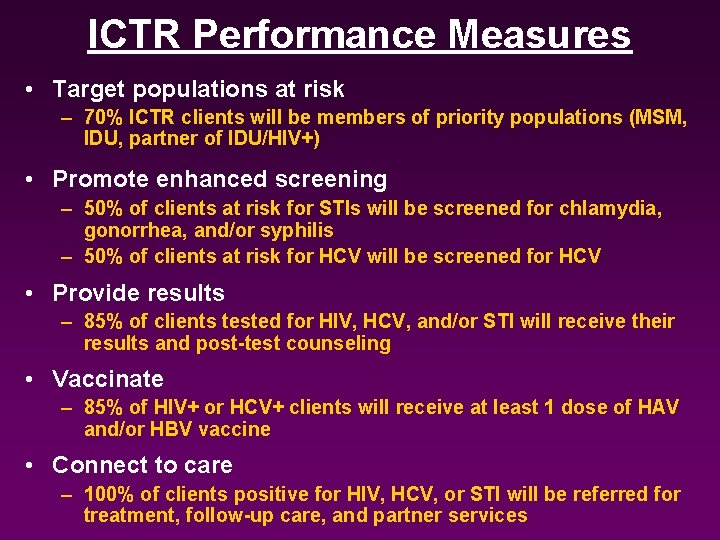
ICTR Performance Measures • Target populations at risk – 70% ICTR clients will be members of priority populations (MSM, IDU, partner of IDU/HIV+) • Promote enhanced screening – 50% of clients at risk for STIs will be screened for chlamydia, gonorrhea, and/or syphilis – 50% of clients at risk for HCV will be screened for HCV • Provide results – 85% of clients tested for HIV, HCV, and/or STI will receive their results and post-test counseling • Vaccinate – 85% of HIV+ or HCV+ clients will receive at least 1 dose of HAV and/or HBV vaccine • Connect to care – 100% of clients positive for HIV, HCV, or STI will be referred for treatment, follow-up care, and partner services

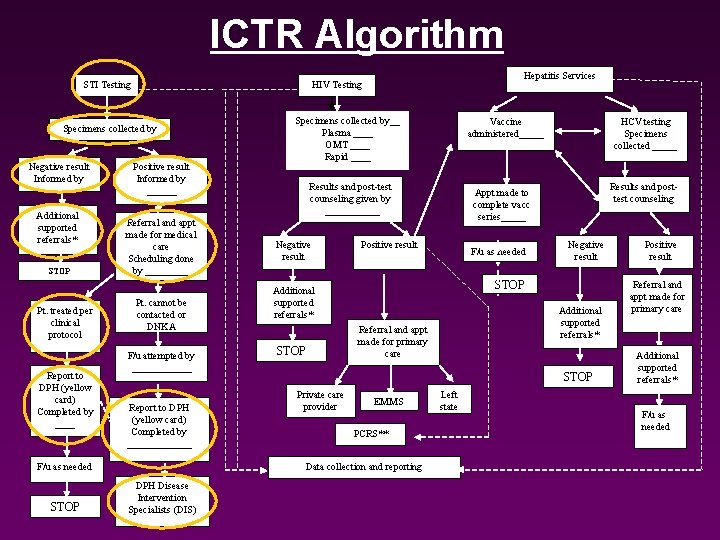
ICTR Algorithm STI Testing Specimens collected by ______ Negative result Informed by Additional supported referrals* STOP Pt. treated per clinical protocol Report to DPH (yellow card) Completed by ____ Positive result Informed by ______ Referral and appt made for medical care Scheduling done by _____ Pt. cannot be contacted or DNKA F/u attempted by ______ Report to DPH (yellow card) Completed by _______ Specimens collected by__ Plasma ____ OMT ____ Rapid ____ Negative result DPH Disease Intervention Specialists (DIS) Results and posttest counseling Appt made to complete vacc series_____ Positive result F/u as needed Negative result STOP Additional supported referrals* STOP HCV testing Specimens collected _____ Vaccine administered_____ Results and post-test counseling given by ______ Additional supported referrals* Referral and appt made for primary care STOP Private care provider EMMS PCRS** Data collection and reporting F/u as needed STOP Hepatitis Services HIV Testing Left state Positive result Referral and appt made for primary care Additional supported referrals* F/u as needed

Training and Technical Assistance • Site Visits – Develop clinic-specific protocols for counseling, testing, specimen collection/submission based on algorithm • Training and support – Site-by site training on relationship between HIV, STIs, viral hepatitis – STI training focused on: • Basic information on chlamydia, gonorrhea, and syphilis • STI related counseling and risk reduction messages • Specimen collection and submission (if applicable)

ICTR: HIV Testing Data For 4/1/07 -12/31/07 Total HIV Tests 11, 123 Gender Male 6170 (56%) Female 4915 (44%) Missing 38 (<1%) Mean Age 34. 6 years Total Positives 110 (1%)

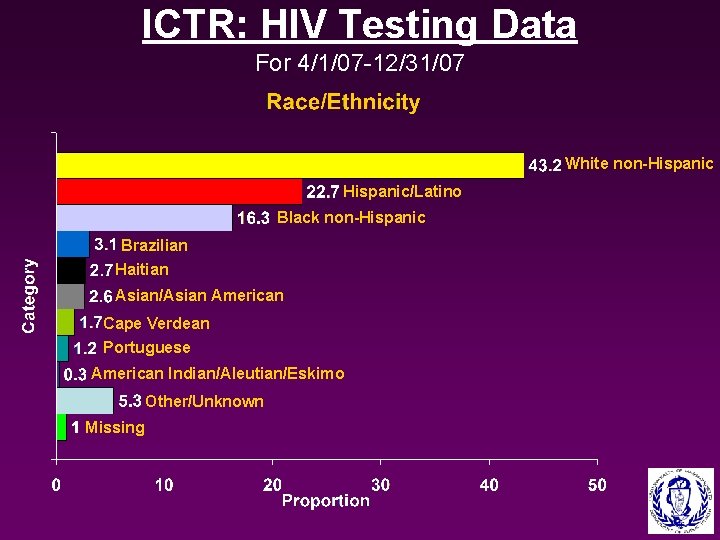
ICTR: HIV Testing Data For 4/1/07 -12/31/07 White non-Hispanic/Latino Black non-Hispanic Brazilian Haitian Asian/Asian American Cape Verdean Portuguese American Indian/Aleutian/Eskimo Other/Unknown Missing

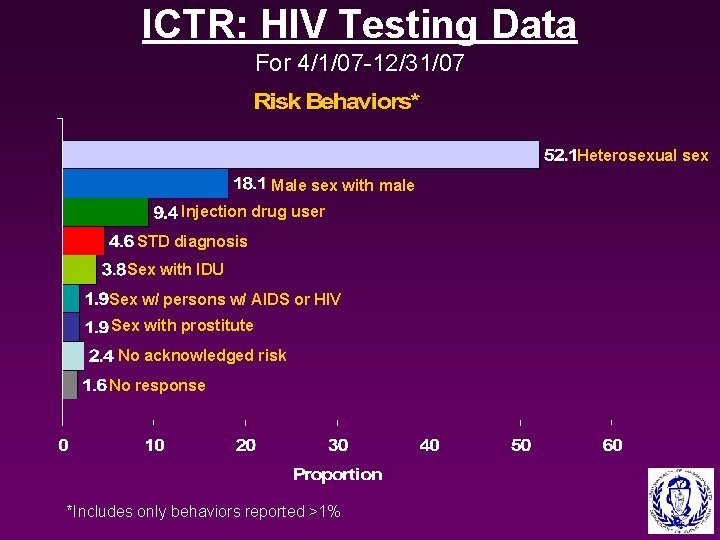
ICTR: HIV Testing Data For 4/1/07 -12/31/07 Heterosexual sex Male sex with male Injection drug user STD diagnosis Sex with IDU Sex w/ persons w/ AIDS or HIV Sex with prostitute No acknowledged risk No response *Includes only behaviors reported >1%

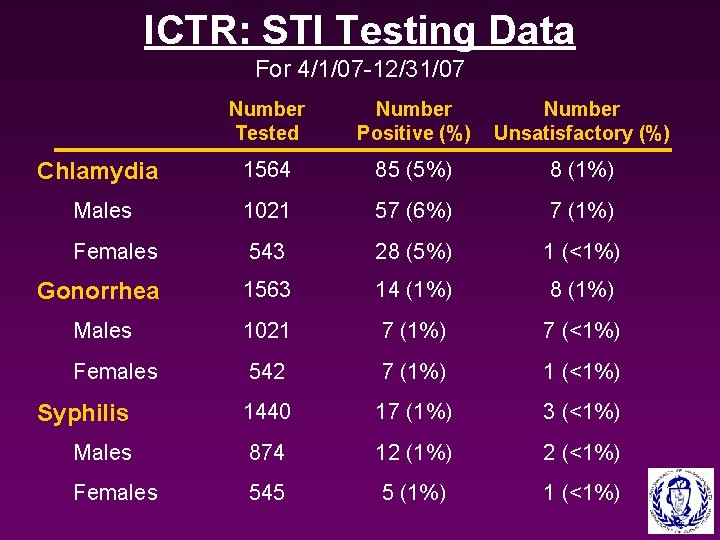
ICTR: STI Testing Data For 4/1/07 -12/31/07 Number Tested Number Positive (%) Number Unsatisfactory (%) Chlamydia 1564 85 (5%) 8 (1%) Males 1021 57 (6%) 7 (1%) Females 543 28 (5%) 1 (<1%) Gonorrhea 1563 14 (1%) 8 (1%) Males 1021 7 (1%) 7 (<1%) Females 542 7 (1%) 1 (<1%) Syphilis 1440 17 (1%) 3 (<1%) Males 874 12 (1%) 2 (<1%) Females 545 5 (1%) 1 (<1%)

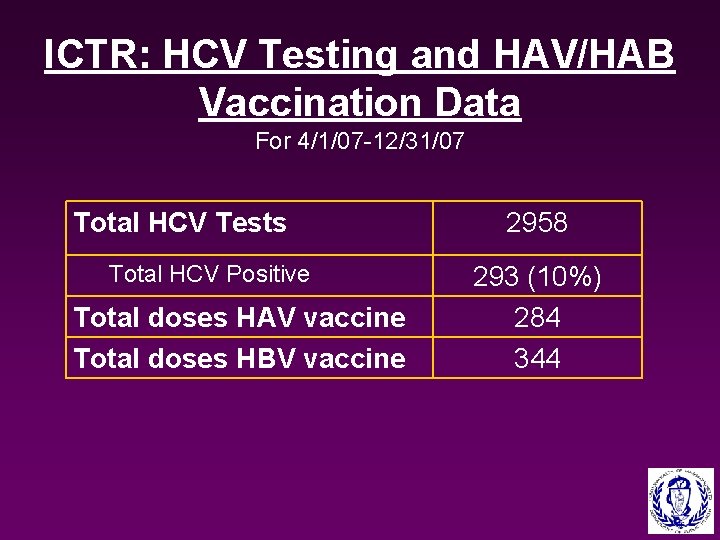
ICTR: HCV Testing and HAV/HAB Vaccination Data For 4/1/07 -12/31/07 Total HCV Tests Total HCV Positive Total doses HAV vaccine Total doses HBV vaccine 2958 293 (10%) 284 344

ICTR: Challenges • Data collection and analysis • Access to clinical staff at ICTR sites for vaccination • Reimbursement for care for STIpositive or HCV-positive clients

Future Directions: Revisiting ICTR Performance Measures q Target populations at risk q 70% ICTR clients will be members of priority populations (MSM, IDU, partner of IDU/HIV+) q Promote enhanced screening q 50% of clients at risk for STIs will be screened for chlamydia, gonorrhea, and/or syphilis q 50% of clients at risk for HCV will be screened for HCV q Provide results q 85% of clients tested for HIV, HCV, and/or STI will receive their results and post-test counseling q Vaccinate q 85% of HIV+ or HCV+ clients will receive at least 1 dose of HAV and/or HBV vaccine q Connect to care q 100% of clients positive for HIV, HCV, or STI will be referred for treatment, follow-up care, and partner services

ICTR: Conclusions • Service integration is a viable way to enhance access to STI, HIV, and viral hepatitis testing, prevention, and care • Successful integration requires: – Coordination and active participation at the level of the Department of Public Health – Training and technical support for ICTR providers – New provider relationships with primary care and medical management

Acknowledgements The MDPH ICTR Workgroup: Juliet Berk Brenda Cole Dan Church Joanne De. Vries Bernadette Green Daniel Cohen Lisa Ehle Dawn Fukuda Clare O’Donoghue David Lessard David Novak Additional thanks: Alan Borne Katherine Hsu Debbie Isenberg Support for this project was provided by the MDPH HIV/AIDS Bureau, the MDPH Division of STD Prevention, and the MDPH Viral Hepatitis Program