Purpose of Safety Reporting The rights safety and




























- Slides: 39


Purpose of Safety Reporting ± The rights, safety, and well-being of the trial subjects are the most important considerations and should prevail over interests of science and society(ICH GCP 2. 3). Human Being







Investigator ± All SAEs should be reported immediately to the sponsor except for those SAEs that the protocol or other document identifies as not needing immediate reporting.

Sponsor ± The sponsor should promptly notify all concerned investigator/institution and the regulatory authority of findings that could affect adversely the safety of subjects, impact the conduct of the trial, or alter the IRB/IEC’s approval /favorable opinion to continue the trial

Requisites for Safety Reporting

Grades of AEs ± Using the following general guidelines 0 : No AEs or WNL 1 : Mild AEs 2 : Moderate AEs 3 : Severe AEs 4 : Life-threatening or disabling AEs 5 : Death

Intensity of Adverse Event ± Requiring assessment by the investigator Mild : Non-specific or not altering normal activities Moderate : Causing difficulty performing some activities Severe : Loss of ability to perform activities or requiring intensive treatment

Causality Evaluation ± Exposure ± Time Course ± Likely Cause ± Dechallenge ± Rechallenge Investigator’s Decision

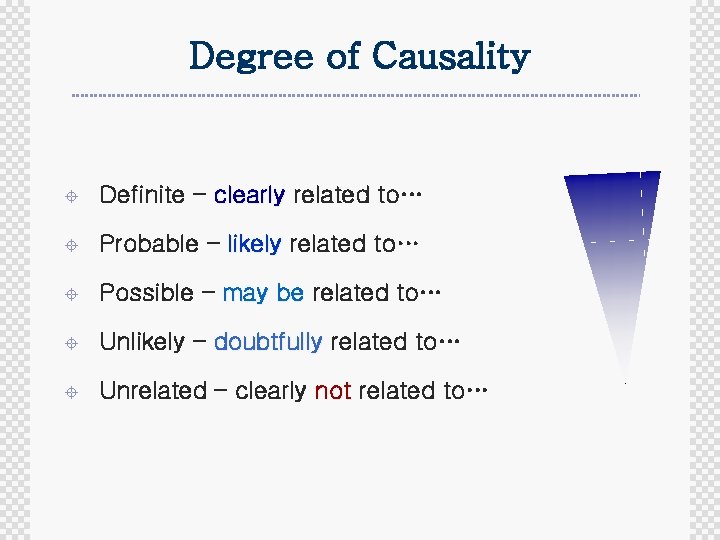
Degree of Causality ± Definite – clearly related to… ± Probable – likely related to… ± Possible – may be related to… ± Unlikely – doubtfully related to… ± Unrelated – clearly not related to…

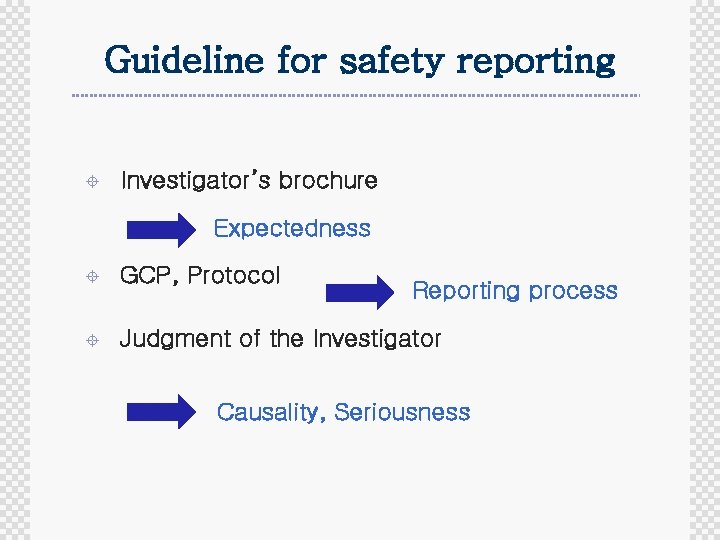
Guideline for safety reporting ± Investigator’s brochure Expectedness ± GCP, Protocol ± Judgment of the Investigator Reporting process Causality, Seriousness

Essential Items in SADR reporting ± Patient Details ± Suspected Medicinal Product(s) ± Other Treatment ± Detail of Suspected ADRs ± Reporter ± Sponsor/Company Details

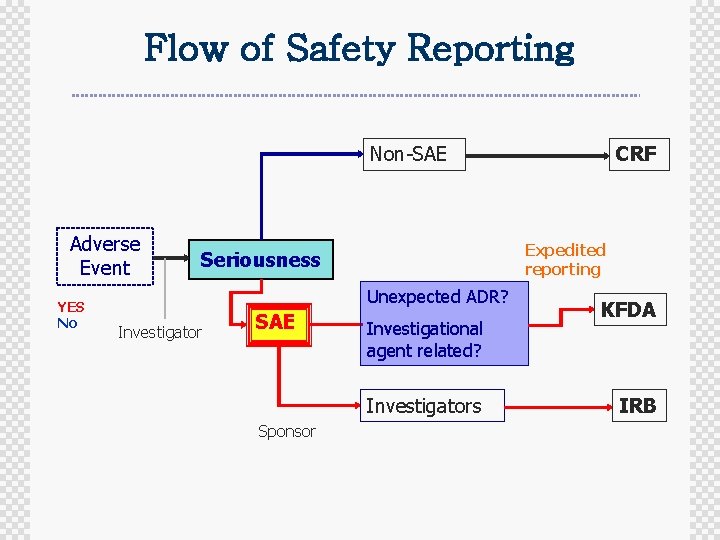
Flow of Safety Reporting Non-SAE Adverse Event YES No Expedited reporting Seriousness Unexpected ADR? Investigator SAE Investigational agent related? Investigators Sponsor CRF KFDA IRB

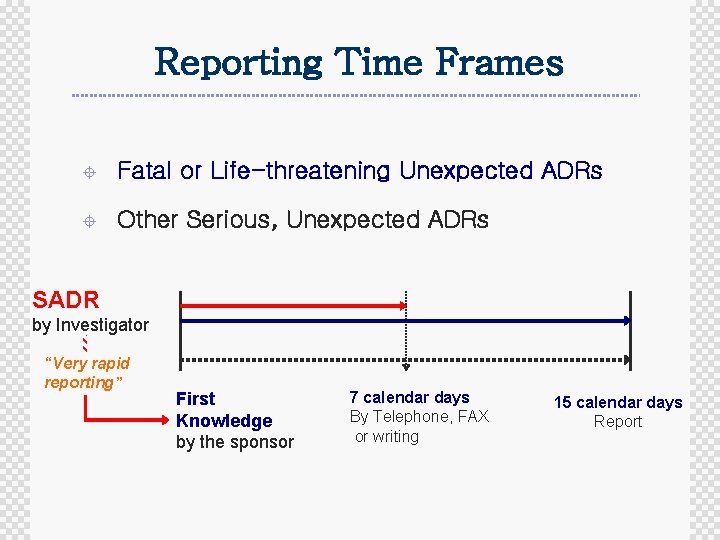
Reporting Time Frames ± Fatal or Life-threatening Unexpected ADRs ± Other Serious, Unexpected ADRs SADR by Investigator “Very rapid reporting” First Knowledge by the sponsor 7 calendar days By Telephone, FAX or writing 15 calendar days Report

Practice of Safety Reporting












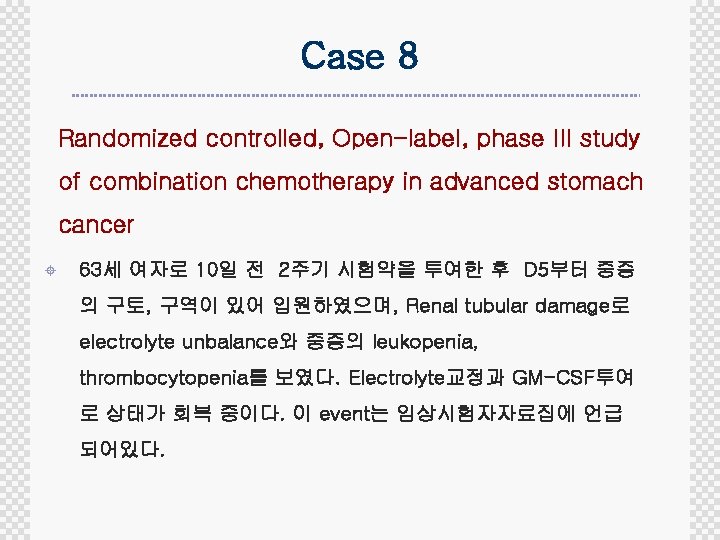
Case 8 Randomized controlled, Open-label, phase III study of combination chemotherapy in advanced stomach cancer ± 63세 여자로 10일 전 2주기 시험약을 투여한 후 D 5부터 중증 의 구토, 구역이 있어 입원하였으며, Renal tubular damage로 electrolyte unbalance와 중증의 leukopenia, thrombocytopenia를 보였다. Electrolyte교정과 GM-CSF투여 로 상태가 회복 중이다. 이 event는 임상시험자자료집에 언급 되어있다.

SADR reporting form(1) CMC-2003 1945/03/21 58 174 58. 6

SADR reporting form (2) 발열성 호중구감소증 폐렴 03/06 03/12 2 4 1 3

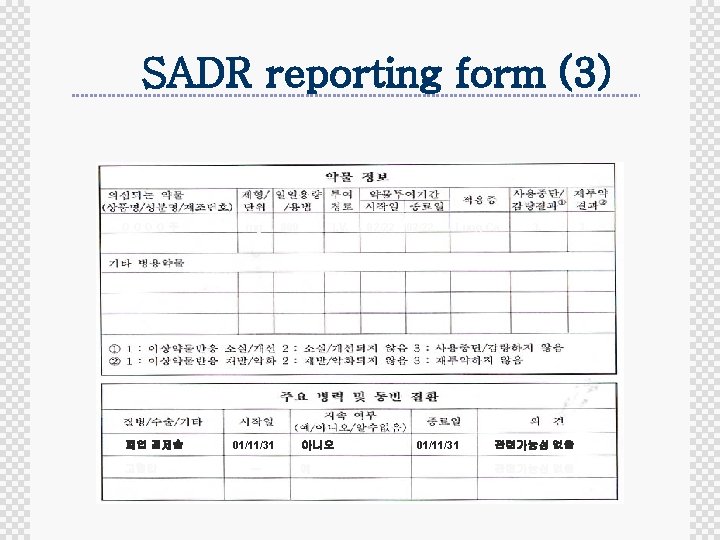
SADR reporting form (3) OOOO주 폐엽 절제술 고혈압 mg 01/11/31 ㅡ 800 I. V. 아니오 예 02/22 Lung Ca 01/11/31 3. 관련가능성 없음 3.

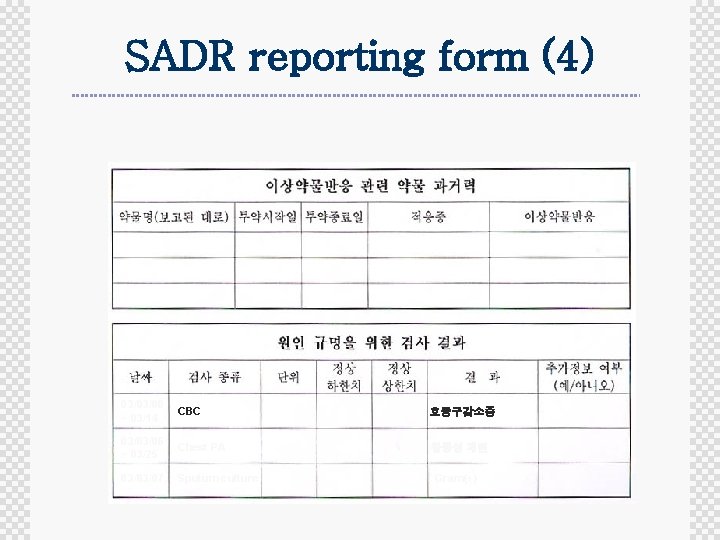
SADR reporting form (4) 03/03/06 ~ 03/14 CBC 호중구감소증 03/03/06 ~ 03/25 Chest PA 활동성 폐렴 03/03/07 Sputum culture Gram(+)

SADR reporting form (5)


The ways of encouraging safety reporting ± Investigator education ® Understand how to monitoring and reporting ® Know what AEs to expect ± Subject education ® Ask investigator’s advice on everything ® Use of patient diary
 Ind safety report
Ind safety report Positive rights and negative rights
Positive rights and negative rights Moral duties
Moral duties Legal rights vs moral rights
Legal rights vs moral rights Negative right
Negative right Littorial rights
Littorial rights Negative rights
Negative rights Positive vs negative rights
Positive vs negative rights Negative rights vs positive rights
Negative rights vs positive rights Purpose of financial statements
Purpose of financial statements Accenture delivery suits
Accenture delivery suits Accenture delivery tools for reporting
Accenture delivery tools for reporting Duke safety reporting system
Duke safety reporting system Patient safety incident reporting form
Patient safety incident reporting form Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Chụp tư thế worms-breton
Chụp tư thế worms-breton Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia Kể tên các môn thể thao
Kể tên các môn thể thao Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Cong thức tính động năng
Cong thức tính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế chỉ nói điều hay thôi
Cái miệng nó xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thế nào là giọng cùng tên?
Thế nào là giọng cùng tên? Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phối cảnh
Phối cảnh































































