Purification of rare earth elements for lowbackground experiments

Purification of rare earth elements for low-background experiments Roman Boiko National University of Life and Environmental Sciences of Ukraine, Institute for Nuclear Research, Kyiv, Ukraine

outline • Introduction • Rare earth elements – What are rare earth elements? – Properties – Technology • Purification of REE at LNGS – Purification of Nd and Gd – Purification of Ce • Conclusions 2

Introduction • Double beta decay is one of the top scientific interest in modern astroparticle physics • There are many potentially 2 active isotopes among the rare earth elements (cerium, neodymium, samarium, gadolinium, dysprosium, erbium and ytterbium) • The most interesting are 136 Ce, 152, 160 Gd and 150 Nd • Such a kind of experiments requires low level of background (the lower, the better) • All materials and even chemically individual compounds are always mixture. Original source of all impurities is nature (subsoil resources, air) The most dangerous radio nuclei are K, Rb, Cs – Ra – Ac, La, Sm, Lu – Th, U • Purification of materials and compounds is important stage in low counting experiments 3
Introduction Purity grades of some chemicals There a lot of different purity grades of chemicals in different countries and companies Sigma-Aldrich® standard product quality grades: Technical (purity may be <90%) Reagent. Plus® (purity is ≥ 95%) ACS reagent (American Chemical Society standards) puriss p. a. , ACS reagent (exceeds ACS standards) % purity (defined as a percent purity) Trace. SELECT® (metal traces are below 0. 01 ppm) Trace. SELECT® Ultra (trace impurities are below <0. 1 ppb) Many chemicals are standardised for a field of utilization: Chromatography, Medicine, Food, Optics, Electronics, etc Impossible to find chemicals with standardized radio-purity (pig in a poke) 4
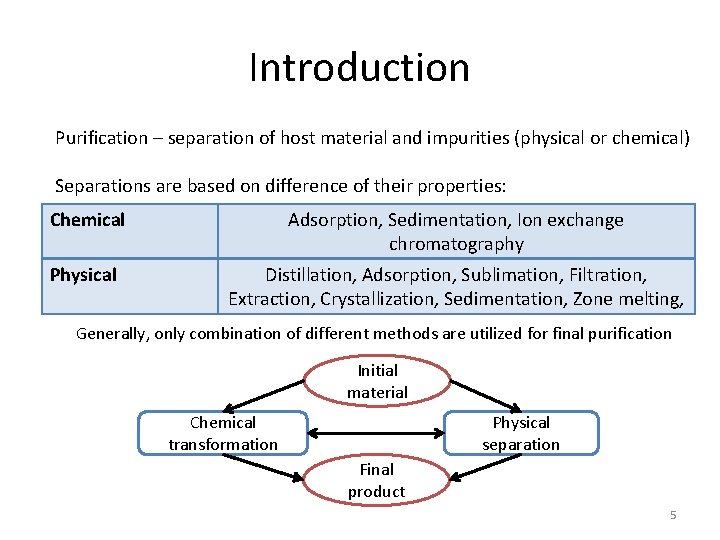
Introduction Purification – separation of host material and impurities (physical or chemical) Separations are based on difference of their properties: Chemical Adsorption, Sedimentation, Ion exchange chromatography Physical Distillation, Adsorption, Sublimation, Filtration, Extraction, Crystallization, Sedimentation, Zone melting, Generally, only combination of different methods are utilized for final purification Initial material Chemical transformation Physical separation Final product 5
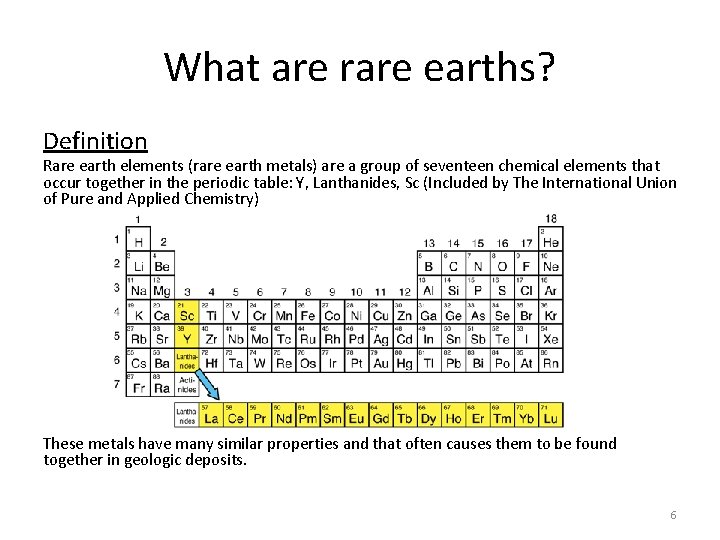
What are rare earths? Definition Rare earth elements (rare earth metals) are a group of seventeen chemical elements that occur together in the periodic table: Y, Lanthanides, Sc (Included by The International Union of Pure and Applied Chemistry) These metals have many similar properties and that often causes them to be found together in geologic deposits. 6
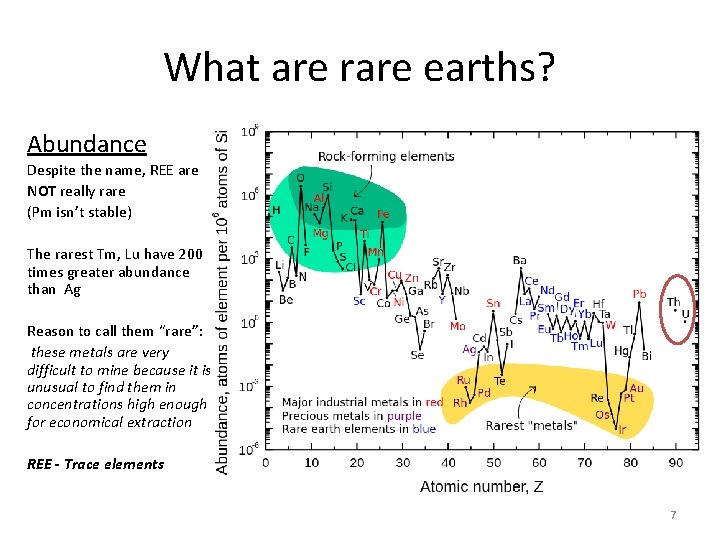
What are rare earths? Abundance Despite the name, REE are NOT really rare (Pm isn’t stable) The rarest Tm, Lu have 200 times greater abundance than Ag Reason to call them “rare”: these metals are very difficult to mine because it is unusual to find them in concentrations high enough for economical extraction REE - Trace elements 7

What are rare earths? Mineral Resources There are more than 200 rare earth bearing minerals. But only some of them are used to REE enrichment and production: Apatite, cheralite, eudialyte, loparite, phosphorites, rare-earth-bearing (ion adsorption) clays, secondary monazite, spent uranium solutions, and xenotime Economically exploited minerals are monazite and bastnaesite. Monazite – phosphate mineral containing rare earth metals – – Monazite-(Ce), (Ce, La, Nd, Th)PO 4 (the most common member) 4 -12% Th. O 2 Monazite-(La), (La, Ce, Nd)PO 4 Monazite-(Nd), (Nd, La, Ce)PO 4 Monazite-(Sm), (Sm, Gd, Ce, Th)PO 4 Bastnaesite – carbonate-fluoride minerals – Bastnaesite-(Ce), (Ce, La)CO 3 F – Bastnaesite-(La), (La, Ce)CO 3 F – Bastnaesite-(Y), (Y, Ce)CO 3 F 8
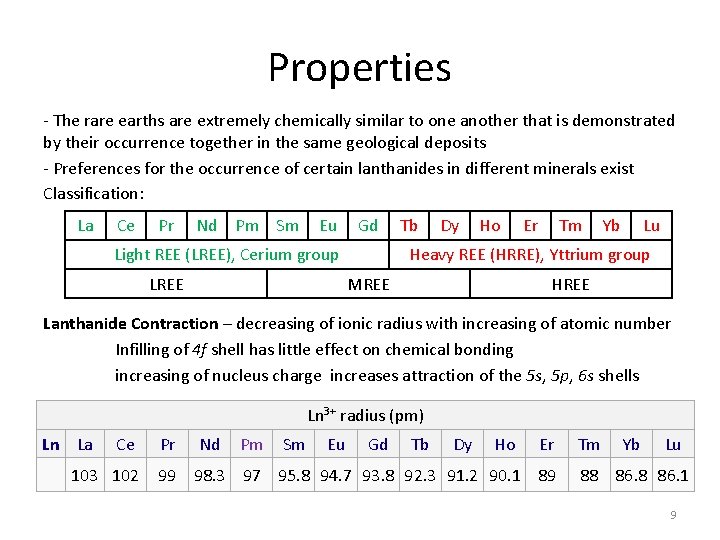
Properties - The rare earths are extremely chemically similar to one another that is demonstrated by their occurrence together in the same geological deposits - Preferences for the occurrence of certain lanthanides in different minerals exist Classification: La Ce Pr Nd Pm Sm Eu Gd Light REE (LREE), Cerium group Tb Dy Ho Er Tm Yb Lu Heavy REE (HRRE), Yttrium group LREE MREE HREE Lanthanide Contraction – decreasing of ionic radius with increasing of atomic number Infilling of 4 f shell has little effect on chemical bonding increasing of nucleus charge increases attraction of the 5 s, 5 p, 6 s shells Ln 3+ radius (pm) Ln La Ce 103 102 Pr Nd Pm Sm Eu Gd Tb Dy Ho Er 99 98. 3 97 95. 8 94. 7 93. 8 92. 3 91. 2 90. 1 89 Tm Yb Lu 88 86. 1 9
![Properties Atomic electron configuration (all begin with [Xe]) 5 d 1 6 s 2 Properties Atomic electron configuration (all begin with [Xe]) 5 d 1 6 s 2](http://slidetodoc.com/presentation_image_h/c406ca6818ad3fcbe6d786112f88e154/image-10.jpg)
Properties Atomic electron configuration (all begin with [Xe]) 5 d 1 6 s 2 4 f 1 4 f 3 1 5 d 6 s 2 2 6 s 4 f 4 6 s 2 4 f 5 6 s 2 4 f 6 6 s 2 4 f 7 4 f 9 1 5 d 6 s 2 2 6 s 4 f 10 6 s 2 4 f 11 6 s 2 4 f 12 6 s 2 4 f 13 6 s 2 4 f 14 5 d 1 6 s 2 Oxidation states +2 +3 +4 La Ce Pr Nd Pm Sm Eu Yb Gd Tb Dy Ho Er Tm Yb Lu Tb 6 Ln + 3 O 2 = 2 Ln 2 O 3 Ce + O 2 = Ce. O 2 12 Pr +11 O 2 = 2 Pr 6 O 11 8 Tb + 7 O 2 = 2 Tb 4 O 7 10
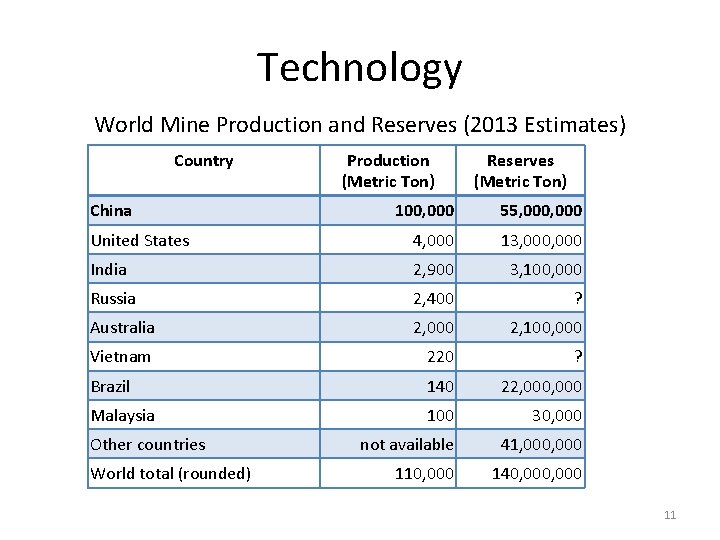
Technology World Mine Production and Reserves (2013 Estimates) Country China Production (Metric Ton) Reserves (Metric Ton) 100, 000 55, 000 United States 4, 000 13, 000 India 2, 900 3, 100, 000 Russia 2, 400 ? Australia 2, 000 2, 100, 000 Vietnam 220 ? Brazil 140 22, 000 Malaysia 100 30, 000 not available 41, 000 110, 000 140, 000 Other countries World total (rounded) 11
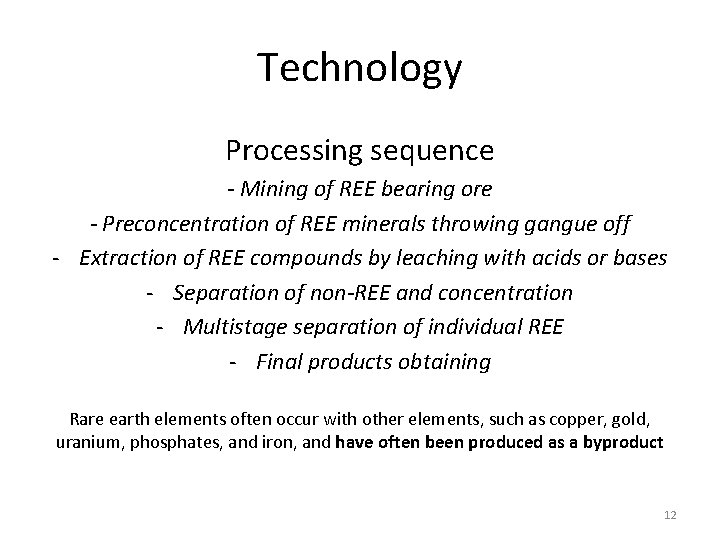
Technology Processing sequence - Mining of REE bearing ore - Preconcentration of REE minerals throwing gangue off - Extraction of REE compounds by leaching with acids or bases - Separation of non-REE and concentration - Multistage separation of individual REE - Final products obtaining Rare earth elements often occur with other elements, such as copper, gold, uranium, phosphates, and iron, and have often been produced as a byproduct 12
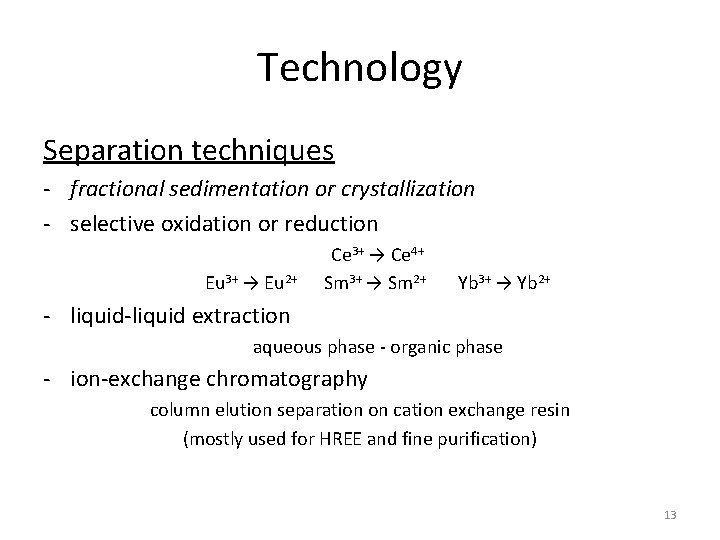
Technology Separation techniques - fractional sedimentation or crystallization - selective oxidation or reduction Ce 3+ → Ce 4+ Eu 3+ → Eu 2+ Sm 3+ → Sm 2+ Yb 3+ → Yb 2+ - liquid-liquid extraction aqueous phase - organic phase - ion-exchange chromatography column elution separation on cation exchange resin (mostly used for HREE and fine purification) 13
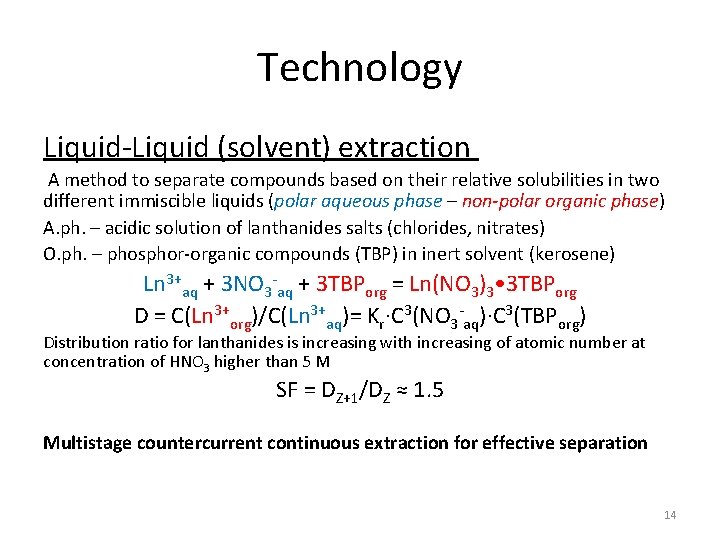
Technology Liquid-Liquid (solvent) extraction A method to separate compounds based on their relative solubilities in two different immiscible liquids (polar aqueous phase – non-polar organic phase) A. ph. – acidic solution of lanthanides salts (chlorides, nitrates) O. ph. – phosphor-organic compounds (TBP) in inert solvent (kerosene) Ln 3+aq + 3 NO 3 -aq + 3 TBPorg = Ln(NO 3)3 • 3 TBPorg D = C(Ln 3+org)/C(Ln 3+aq)= Kr∙C 3(NO 3 -aq)∙C 3(TBPorg) Distribution ratio for lanthanides is increasing with increasing of atomic number at concentration of HNO 3 higher than 5 M SF = DZ+1/DZ ≈ 1. 5 Multistage countercurrent continuous extraction for effective separation 14

Technology Purity grades of REE Metals and oxides – the most common commercial products Specific definition of oxides purity: REO basis (rare earth oxide basis) - content of a specific rare earth oxide in comparison to TREO - total rare earth oxides Label "Ce. O 2 > 99%" often means Ce. O 2/TREO > 99%. Purity = REO/TREO x Part of TREO in the material despite long multistage separation technology, even high purity grade commercial lanthanide compounds contain 238 U, 226 Ra and 232, 228 Th typically on the level of ~ (0. 1 – 1) Bq/kg. 15
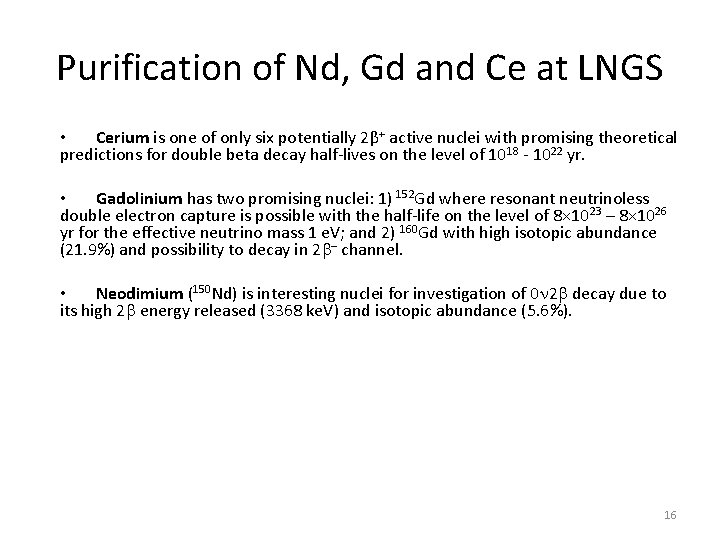
Purification of Nd, Gd and Ce at LNGS • Cerium is one of only six potentially 2β+ active nuclei with promising theoretical predictions for double beta decay half-lives on the level of 1018 - 1022 yr. • Gadolinium has two promising nuclei: 1) 152 Gd where resonant neutrinoless double electron capture is possible with the half-life on the level of 8× 10 23 – 8× 1026 yr for the effective neutrino mass 1 e. V; and 2) 160 Gd with high isotopic abundance (21. 9%) and possibility to decay in 2 – channel. • Neodimium (150 Nd) is interesting nuclei for investigation of 0 2 decay due to its high 2 energy released (3368 ke. V) and isotopic abundance (5. 6%). 16
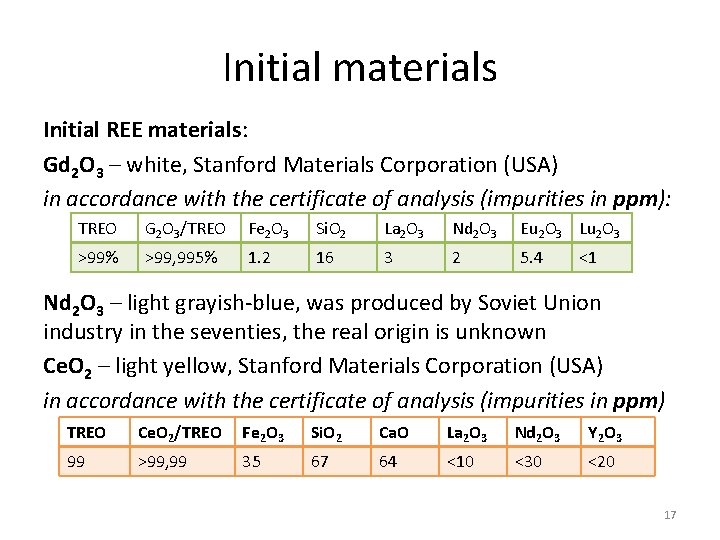
Initial materials Initial REE materials: Gd 2 O 3 – white, Stanford Materials Corporation (USA) in accordance with the certificate of analysis (impurities in ppm): TREO G 2 O 3/TREO Fe 2 O 3 Si. O 2 La 2 O 3 Nd 2 O 3 Eu 2 O 3 Lu 2 O 3 >99% >99, 995% 1. 2 16 3 2 5. 4 <1 Nd 2 O 3 – light grayish-blue, was produced by Soviet Union industry in the seventies, the real origin is unknown Ce. O 2 – light yellow, Stanford Materials Corporation (USA) in accordance with the certificate of analysis (impurities in ppm) TREO Ce. O 2/TREO Fe 2 O 3 Si. O 2 Ca. O La 2 O 3 Nd 2 O 3 Y 2 O 3 99 >99, 99 35 67 64 <10 <30 <20 17
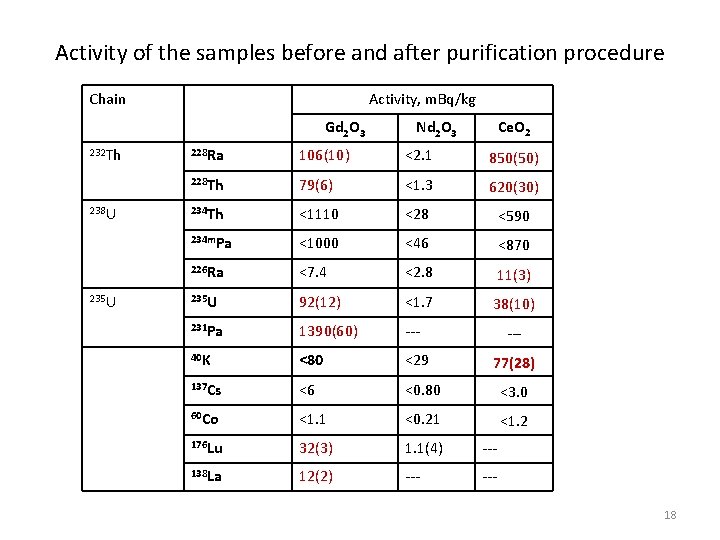
Activity of the samples before and after purification procedure Chain Activity, m. Bq/kg Gd 2 O 3 232 Th 238 U 235 U Nd 2 O 3 Ce. O 2 228 Ra 106(10) <2. 1 850(50) 228 Th 79(6) <1. 3 620(30) 234 Th <1110 <28 <590 234 m. Pa <1000 <46 <870 226 Ra <7. 4 <2. 8 11(3) 235 U 92(12) <1. 7 38(10) 231 Pa 1390(60) --- 40 K <80 <29 77(28) 137 Cs <6 <0. 80 <3. 0 60 Co <1. 1 <0. 21 <1. 2 176 Lu 32(3) 1. 1(4) --- 138 La 12(2) --- --18
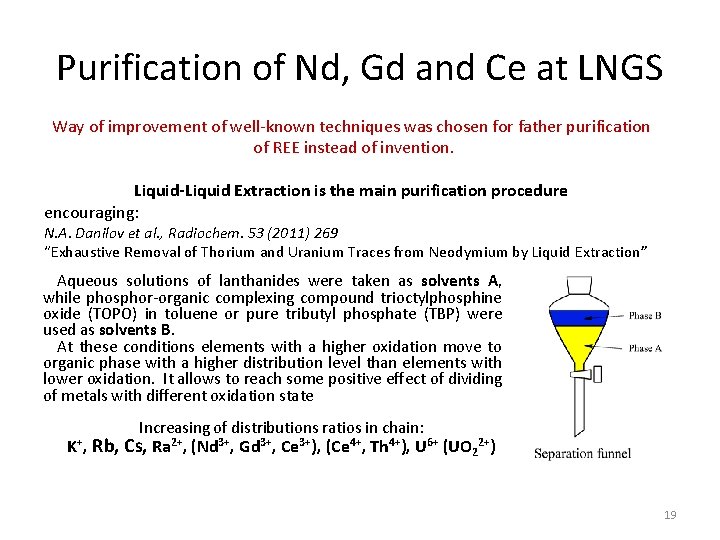
Purification of Nd, Gd and Ce at LNGS Way of improvement of well-known techniques was chosen for father purification of REE instead of invention. Liquid-Liquid Extraction is the main purification procedure encouraging: N. A. Danilov et al. , Radiochem. 53 (2011) 269 “Exhaustive Removal of Thorium and Uranium Traces from Neodymium by Liquid Extraction” Aqueous solutions of lanthanides were taken as solvents A, while phosphor-organic complexing compound trioctylphosphine oxide (TOPO) in toluene or pure tributyl phosphate (TBP) were used as solvents B. At these conditions elements with a higher oxidation move to organic phase with a higher distribution level than elements with lower oxidation. It allows to reach some positive effect of dividing of metals with different oxidation state K+, Increasing of distributions ratios in chain: Rb, Cs, Ra 2+, (Nd 3+, Gd 3+, Ce 3+), (Ce 4+, Th 4+), U 6+ (UO 22+) 19
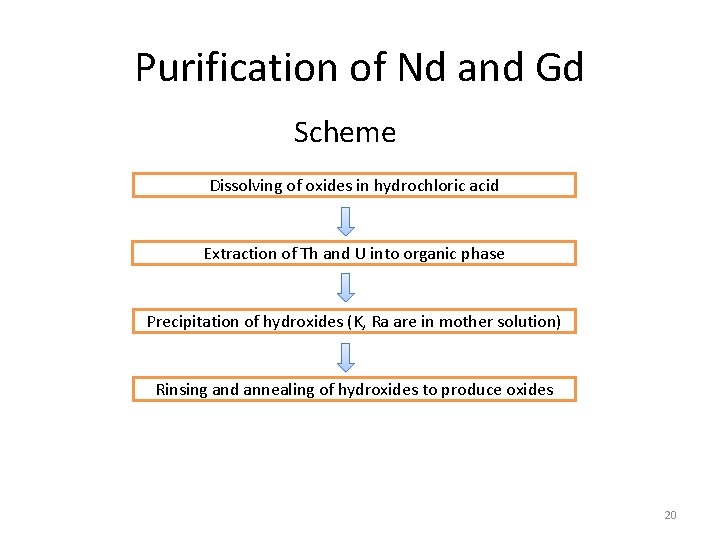
Purification of Nd and Gd Scheme Dissolving of oxides in hydrochloric acid Extraction of Th and U into organic phase Precipitation of hydroxides (K, Ra are in mother solution) Rinsing and annealing of hydroxides to produce oxides 20
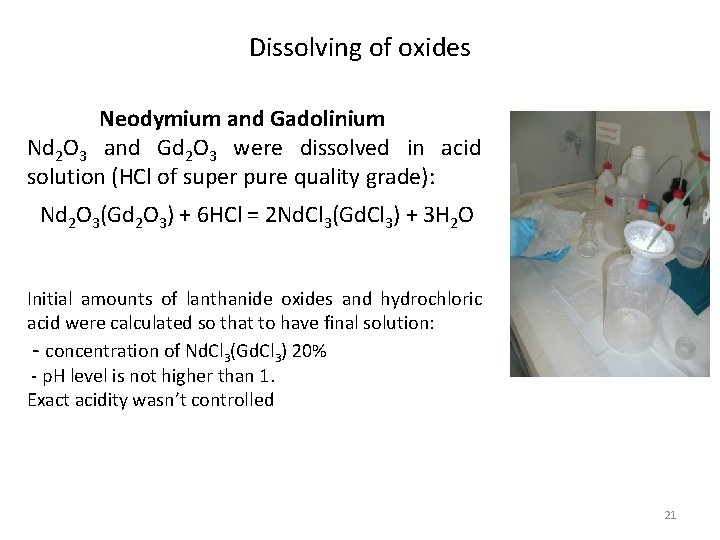
Dissolving of oxides Neodymium and Gadolinium Nd 2 O 3 and Gd 2 O 3 were dissolved in acid solution (HCl of super pure quality grade): Nd 2 O 3(Gd 2 O 3) + 6 HCl = 2 Nd. Cl 3(Gd. Cl 3) + 3 H 2 O Initial amounts of lanthanide oxides and hydrochloric acid were calculated so that to have final solution: - concentration of Nd. Cl 3(Gd. Cl 3) 20% - p. H level is not higher than 1. Exact acidity wasn’t controlled 21
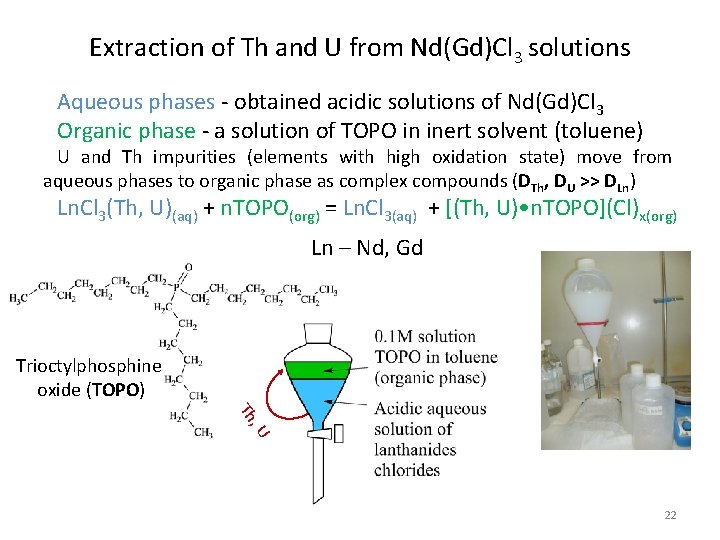
Extraction of Th and U from Nd(Gd)Cl 3 solutions Aqueous phases - obtained acidic solutions of Nd(Gd)Cl 3 Organic phase - a solution of TOPO in inert solvent (toluene) U and Th impurities (elements with high oxidation state) move from aqueous phases to organic phase as complex compounds (DTh, DU >> DLn) Ln. Cl 3(Th, U)(aq) + n. TOPO(org) = Ln. Cl 3(aq) + [(Th, U) • n. TOPO](Cl)x(org) Ln – Nd, Gd Trioctylphosphine oxide (TOPO) , U Th 22
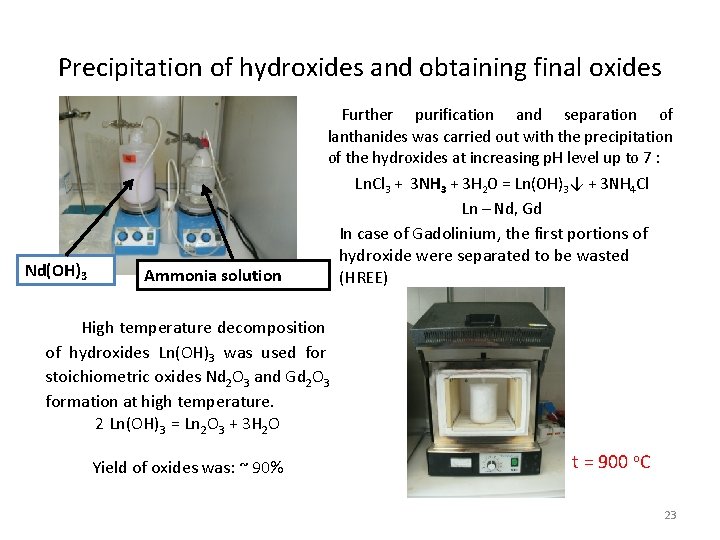
Precipitation of hydroxides and obtaining final oxides Further purification and separation of lanthanides was carried out with the precipitation of the hydroxides at increasing p. H level up to 7 : Ln. Cl 3 + 3 NH 3 + 3 H 2 O = Ln(OH)3↓ + 3 NH 4 Cl Ln – Nd, Gd Nd(OH)3 Ammonia solution In case of Gadolinium, the first portions of hydroxide were separated to be wasted (HREE) High temperature decomposition of hydroxides Ln(OH)3 was used for stoichiometric oxides Nd 2 O 3 and Gd 2 O 3 formation at high temperature. 2 Ln(OH)3 = Ln 2 O 3 + 3 H 2 O Yield of oxides was: ~ 90% t = 900 o. C 23
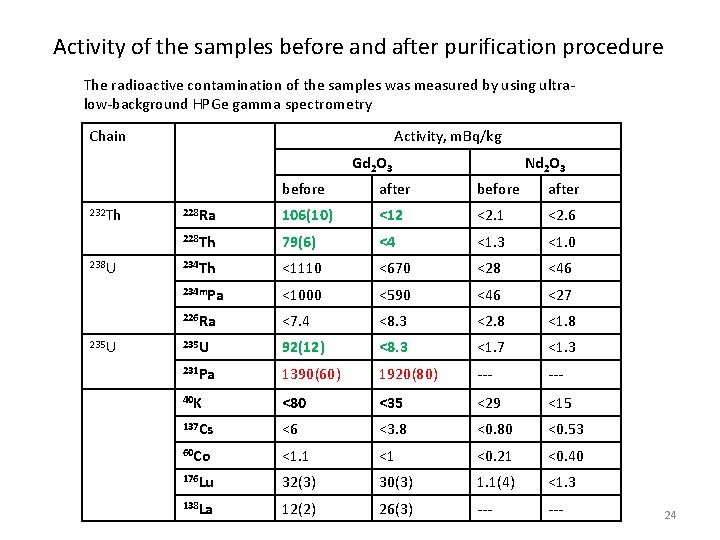
Activity of the samples before and after purification procedure The radioactive contamination of the samples was measured by using ultralow-background HPGe gamma spectrometry Chain Activity, m. Bq/kg Gd 2 O 3 232 Th 238 U 235 U Nd 2 O 3 before after 228 Ra 106(10) <12 <2. 1 <2. 6 228 Th 79(6) <4 <1. 3 <1. 0 234 Th <1110 <670 <28 <46 234 m. Pa <1000 <590 <46 <27 226 Ra <7. 4 <8. 3 <2. 8 <1. 8 235 U 92(12) <8. 3 <1. 7 <1. 3 231 Pa 1390(60) 1920(80) --- 40 K <80 <35 <29 <15 137 Cs <6 <3. 8 <0. 80 <0. 53 60 Co <1. 1 <1 <0. 21 <0. 40 176 Lu 32(3) 30(3) 1. 1(4) <1. 3 138 La 12(2) 26(3) --- 24
Purification of Ce Scheme Dissolving of Ce. O 2 in acid Extraction of Ce into organic phase Re-extraction of Ce into aqueous phase Precipitation of cerium hydroxide Rinsing, drying and annealing of hydroxide 25
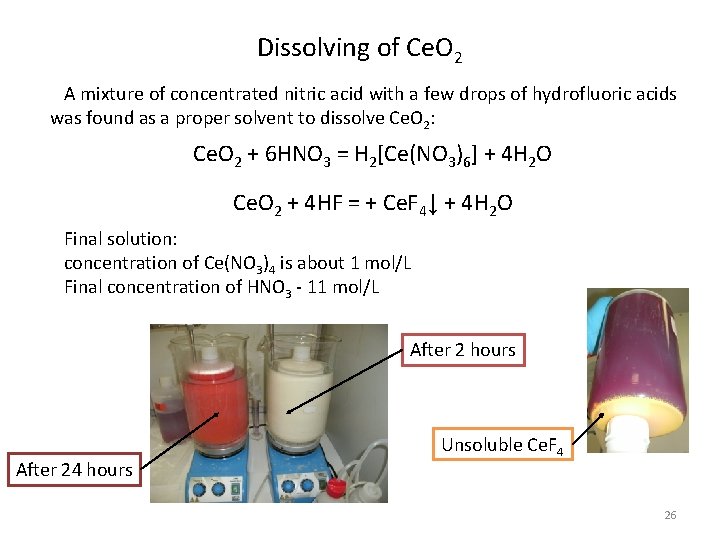
Dissolving of Ce. O 2 A mixture of concentrated nitric acid with a few drops of hydrofluoric acids was found as a proper solvent to dissolve Ce. O 2: Ce. O 2 + 6 HNO 3 = H 2[Ce(NO 3)6] + 4 H 2 O Ce. O 2 + 4 HF = + Ce. F 4↓ + 4 H 2 O Final solution: concentration of Ce(NO 3)4 is about 1 mol/L Final concentration of HNO 3 - 11 mol/L After 2 hours After 24 hours Unsoluble Ce. F 4 26
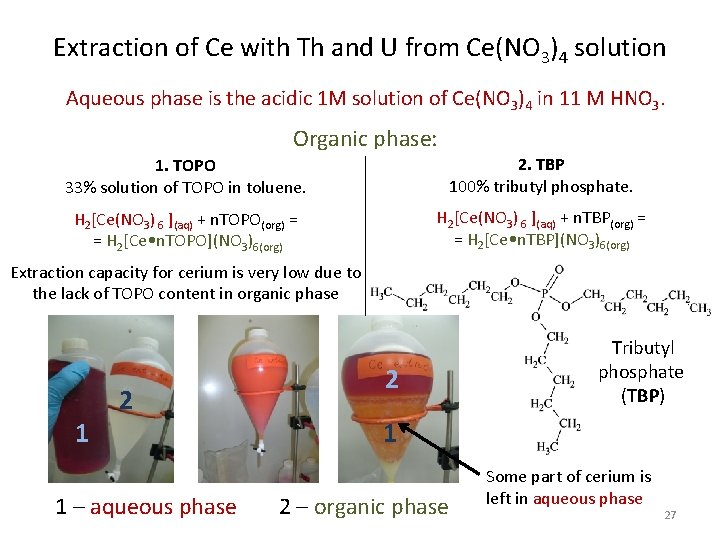
Extraction of Ce with Th and U from Ce(NO 3)4 solution Aqueous phase is the acidic 1 M solution of Ce(NO 3)4 in 11 M HNO 3. Organic phase: 1. TOPO 33% solution of TOPO in toluene. 2. TBP 100% tributyl phosphate. H 2[Ce(NO 3) 6 ](aq) + n. TOPO(org) = = H 2[Ce • n. TOPO](NO 3)6(org) H 2[Ce(NO 3) 6 ](aq) + n. TBP(org) = = H 2[Ce • n. TBP](NO 3)6(org) Extraction capacity for cerium is very low due to the lack of TOPO content in organic phase 1 2 1 – aqueous phase 2 Tributyl phosphate (TBP) 1 2 – organic phase Some part of cerium is left in aqueous phase 27
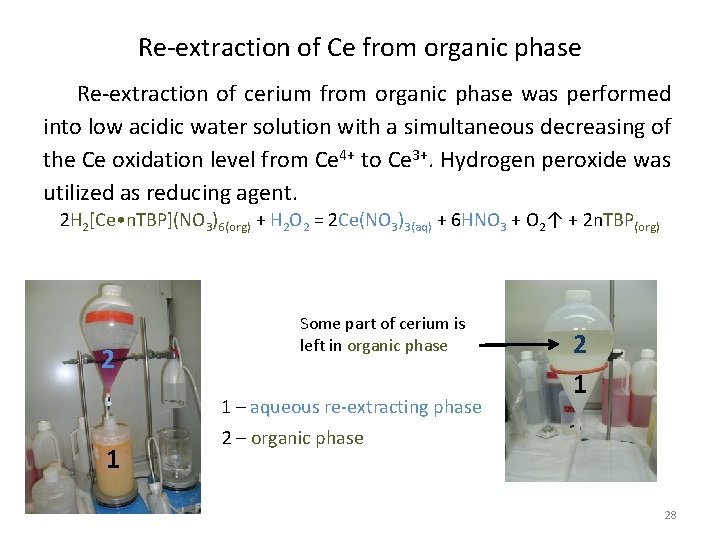
Re-extraction of Ce from organic phase Re-extraction of cerium from organic phase was performed into low acidic water solution with a simultaneous decreasing of the Ce oxidation level from Ce 4+ to Ce 3+. Hydrogen peroxide was utilized as reducing agent. 2 H 2[Ce • n. TBP](NO 3)6(org) + H 2 O 2 = 2 Ce(NO 3)3(aq) + 6 HNO 3 + O 2↑ + 2 n. TBP(org) 2 Some part of cerium is left in organic phase 1 – aqueous re-extracting phase 1 2 – organic phase 28
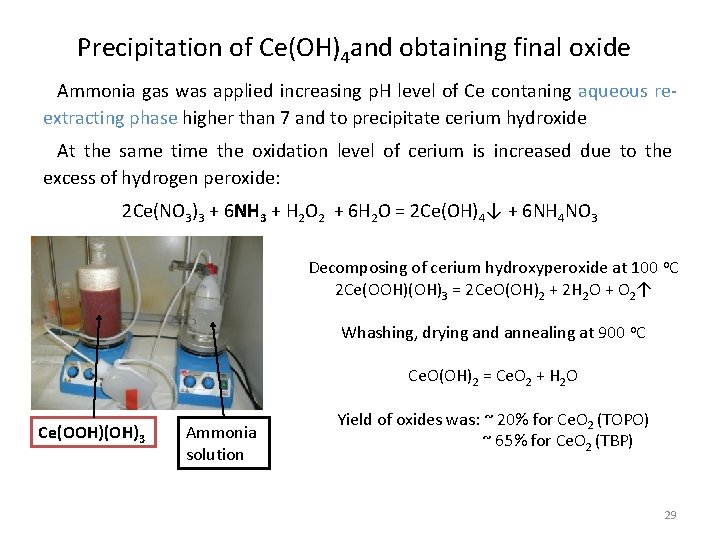
Precipitation of Ce(OH)4 and obtaining final oxide Ammonia gas was applied increasing p. H level of Ce contaning aqueous reextracting phase higher than 7 and to precipitate cerium hydroxide At the same time the oxidation level of cerium is increased due to the excess of hydrogen peroxide: 2 Ce(NO 3)3 + 6 NH 3 + H 2 O 2 + 6 H 2 O = 2 Ce(OH)4↓ + 6 NH 4 NO 3 Decomposing of cerium hydroxyperoxide at 100 o. C 2 Ce(OOH)(OH)3 = 2 Ce. O(OH)2 + 2 H 2 O + O 2↑ Whashing, drying and annealing at 900 o. C Ce. O(OH)2 = Ce. O 2 + H 2 O Ce(OOH)(OH)3 Ammonia solution Yield of oxides was: ~ 20% for Ce. O 2 (TOPO) ~ 65% for Ce. O 2 (TBP) 29
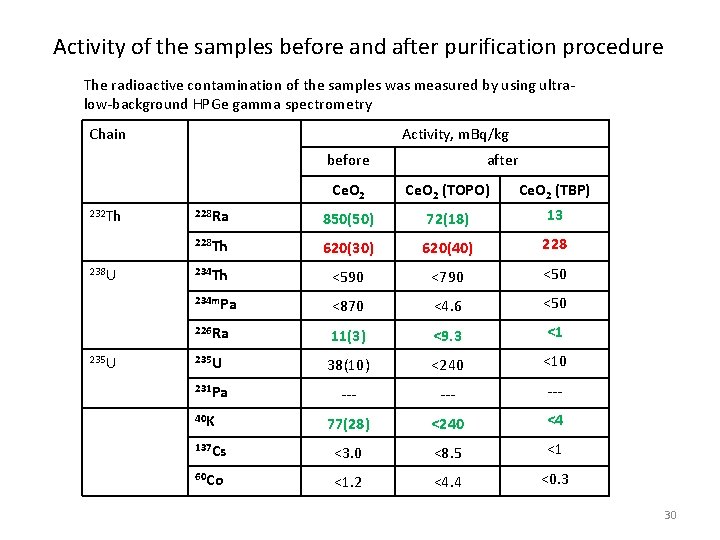
Activity of the samples before and after purification procedure The radioactive contamination of the samples was measured by using ultralow-background HPGe gamma spectrometry Chain Activity, m. Bq/kg before 232 Th 238 U 235 U after Ce. O 2 (TOPO) Ce. O 2 (TBP) 228 Ra 850(50) 72(18) 13 228 Th 620(30) 620(40) 228 234 Th <590 <790 <50 234 m. Pa <870 <4. 6 <50 226 Ra 11(3) <9. 3 <1 235 U 38(10) <240 <10 231 Pa --- --- 77(28) <240 <4 137 Cs <3. 0 <8. 5 <1 60 Co <1. 2 <4. 4 <0. 3 40 K 30
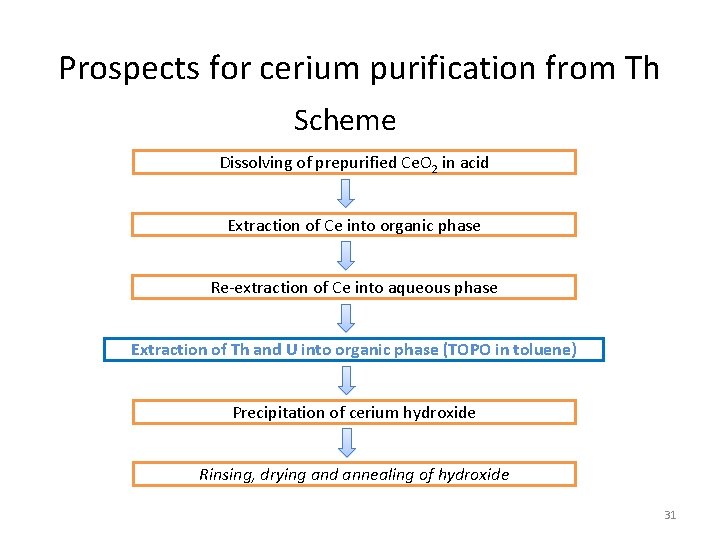
Prospects for cerium purification from Th Scheme Dissolving of prepurified Ce. O 2 in acid Extraction of Ce into organic phase Re-extraction of Ce into aqueous phase Extraction of Th and U into organic phase (TOPO in toluene) Precipitation of cerium hydroxide Rinsing, drying and annealing of hydroxide 31
Conclusions • Modified and improved industrial techniques were applied for father purification of RRE at laboratory conditions • Significant decreasing of Th and U is observed for Nd and Gd purification procedure using solution of TOPO as extractant • Applied purification methods works well for reduction of alkaline (K), alkaline-earth metals (Ra) and uranium in Ce • Additional stage of liquid extraction is required for separation of Th form cerium • Further improvement of liquid extraction and investigation of others methods like ion-exchange chromatography are the nearest tasks for purification 32

Thank you for attention 33
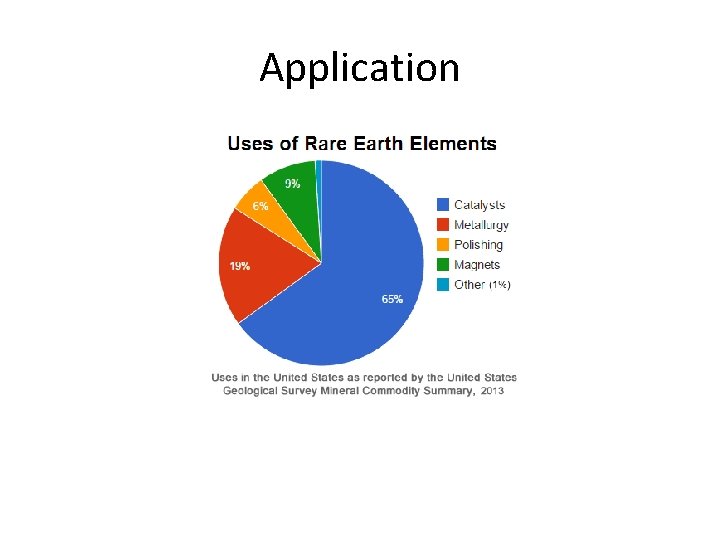
Application
- Slides: 34