Purification of cells from human peripheral blood Mononuclear
Purification of cells from human peripheral blood • Mononuclear cells: Lymphocytes + Monocytes • Polymorphonuclear leukocytes (PMNs) or granulocytes • Platelets or thrombocytes
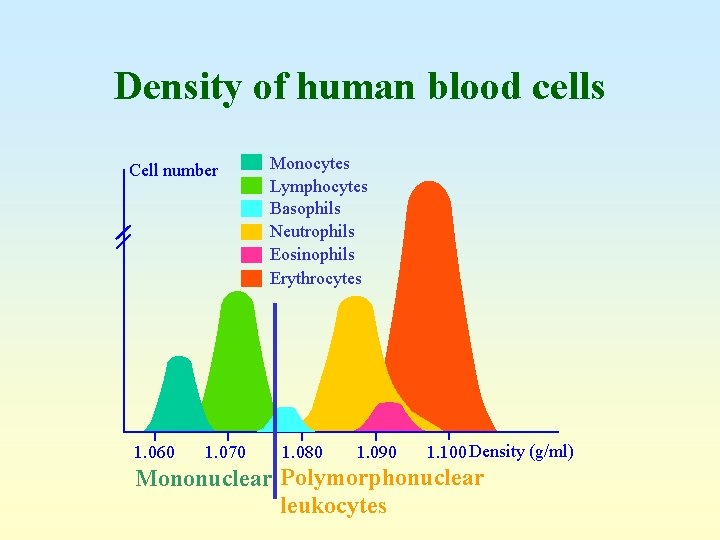
Density of human blood cells Cell number 1. 060 1. 070 Monocytes Lymphocytes Basophils Neutrophils Eosinophils Erythrocytes 1. 080 1. 090 1. 100 Density (g/ml) Mononuclear Polymorphonuclear leukocytes

Isolation of human mononuclear cells I Density barrier method 600 g 15 min 1. 077 g/ml MC RBC PMNs

Axis-Shield Density Gradient Media for isolation of human PBMCs • Lymphoprep™: 9. 1% diatrizoate, 5. 7% polysaccharide; 1. 077 g/ml, 295 m. Osm; endotoxin <0. 13 EU/ml • Nycoprep™ 1. 077: 14. 1% Nycodenz®, 0. 44% Na. Cl, 5 m. M Tricine-Na. OH, p. H 7. 0; 1. 077 g/ml, 295 m. Osm; endotoxin < 0. 13 EU/ml • Optiprep™ (endotoxin < 0. 13 EU/ml) diluted with any suitable balanced salt solution or culture medium to give a 1. 077 g/ml medium (C 03)
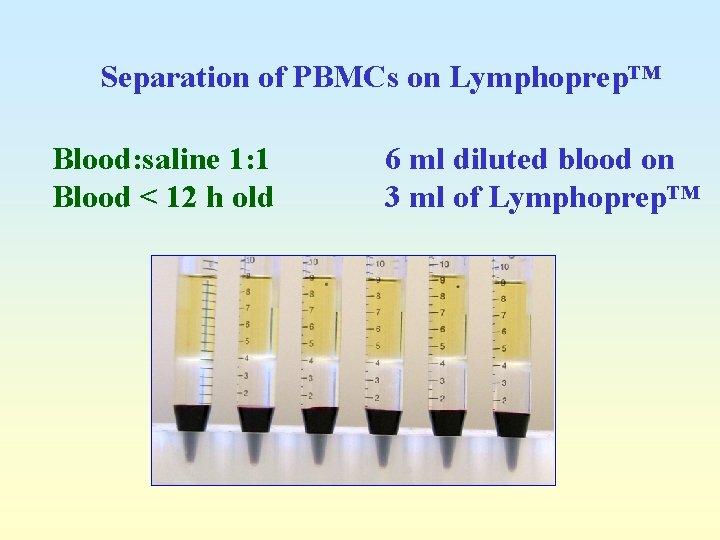
Separation of PBMCs on Lymphoprep™ Blood: saline 1: 1 Blood < 12 h old 6 ml diluted blood on 3 ml of Lymphoprep™

Lymphoprep™ competitors • Ficoll-Paque (GE Health Care (ex Amersham and Pharmacia) • Histopaque 1077 (Sigma) • Not endotoxin tested • More expensive endotoxin-tested versions, e. g. Sigma’s Histopaque 1077 Hybrimax

Lymphoprep™ queries (I) • • • Anticoagulant? Not anticoagulant sensitive Do I have to dilute the blood? Whole blood less easy to layer; poorer % yields Can increase sample: Lymphoprep™ ratio Yes – but not recommended Can I use a leukocyte-rich plasma? Yes, OK to separate PBMCs and PMNs Centrifugation at 4°C rather than room temperature? • Yes, but need to increase time by 5 min

Lymphoprep™ queries (II) • • • Ficoll-Paque or Histopaque work better Impossible – media have identical composition Poor definition of PBMC band at interface Poor layering technique and/or brake problem Poor separation from erythrocytes/PMNs Blood > 12 h old; clinical specimens Can I use it for non-human blood? Maybe primates and some ruminants How can I remove the platelets?

Lymphoprep™ Tube Blood diluted 1: 1 with saline 800 g for 15 min Plastic frit Lymphoprep. TM Medium displaced upwards PBMCs
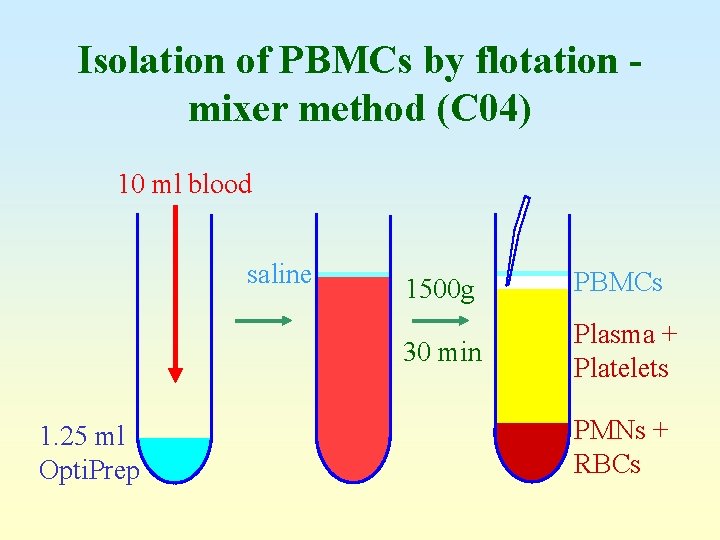
Isolation of PBMCs by flotation mixer method (C 04) 10 ml blood saline 1. 25 ml Opti. Prep 1500 g PBMCs 30 min Plasma + Platelets PMNs + RBCs

Problem with mixer method • • Ratio of cells: plasma (haematocrit value) Normal males: 42 -54% Normal females: 38 -46% Some clinical samples may have significantly lower values
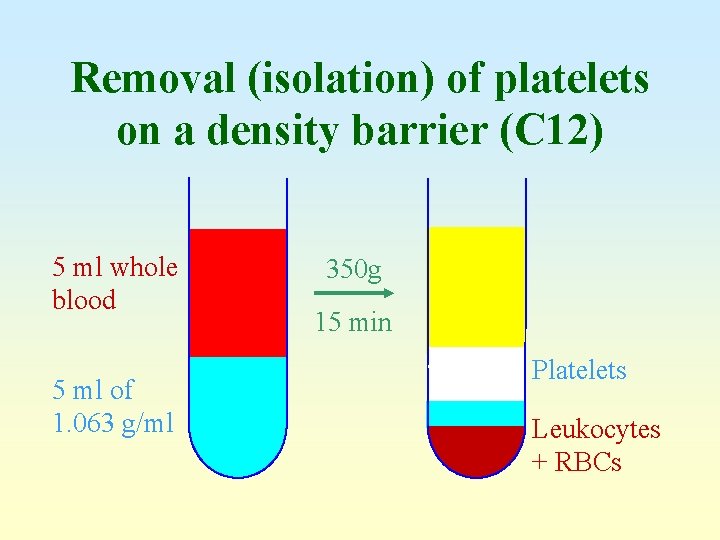
Removal (isolation) of platelets on a density barrier (C 12) 5 ml whole blood 5 ml of 1. 063 g/ml 350 g 15 min Platelets Leukocytes + RBCs

Isolation of platelet-free PBMCs (C 05) saline PBMCs 600 g 1. 077 g/ml Blood + Opti. Prep 1. 095 g/ml 20 min PMNs Plasma + RBCs + Platelets

Axis-Shield Density Gradient Media for the isolation of human PMNs from whole blood • • Polymorphprep™ 13. 8% diatrizoate, 8. 0% dextran 500 Density = 1. 113 g/ml Osmolality = 460 m. Osm
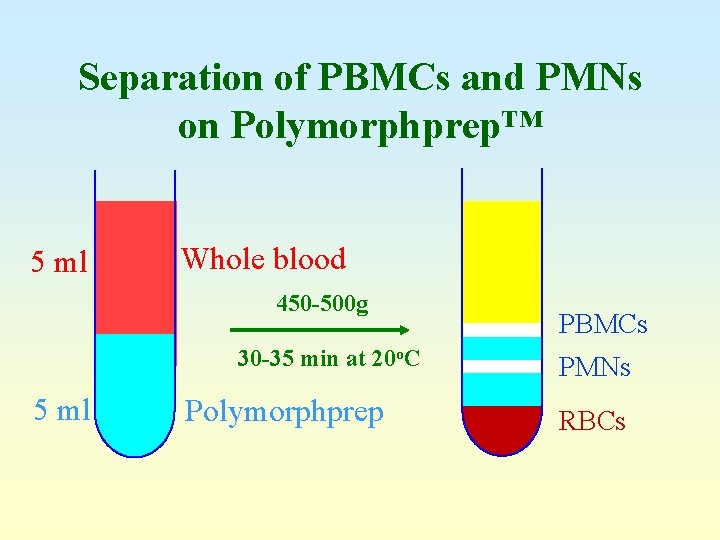
Separation of PBMCs and PMNs on Polymorphprep™ 5 ml Whole blood 450 -500 g 30 -35 min at 20 o. C 5 ml Polymorphprep PBMCs PMNs RBCs
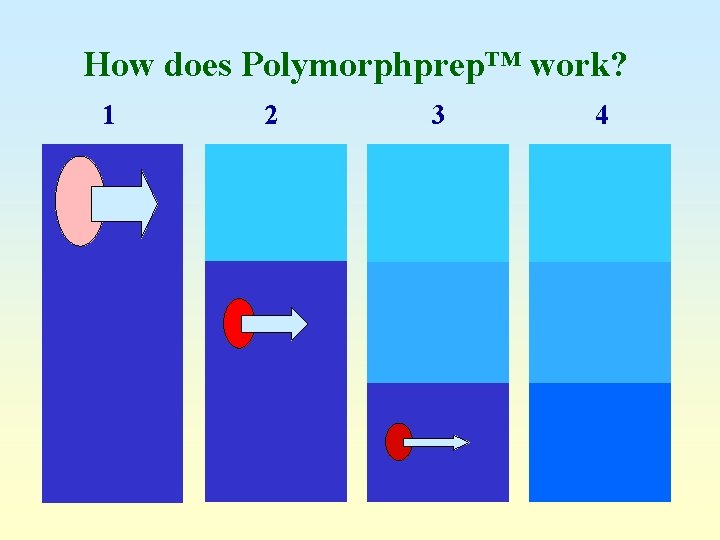
How does Polymorphprep™ work? 1 2 3 4

Polymorphprep™ Separation
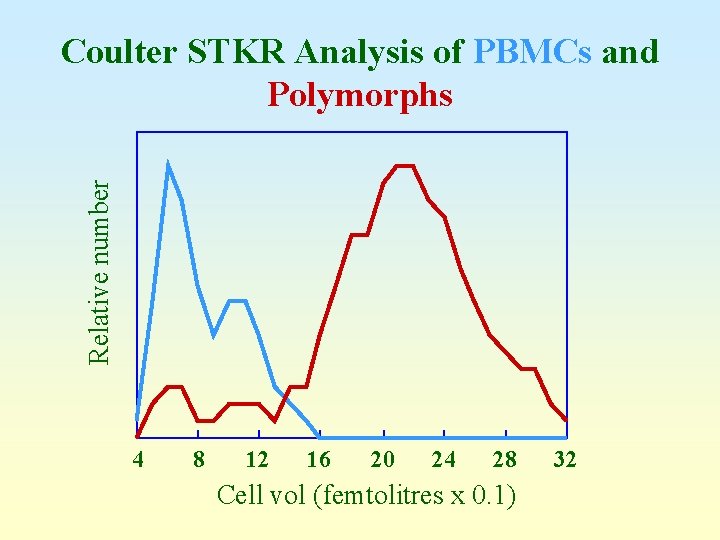
Relative number Coulter STKR Analysis of PBMCs and Polymorphs 4 8 12 16 20 24 28 Cell vol (femtolitres x 0. 1) 32
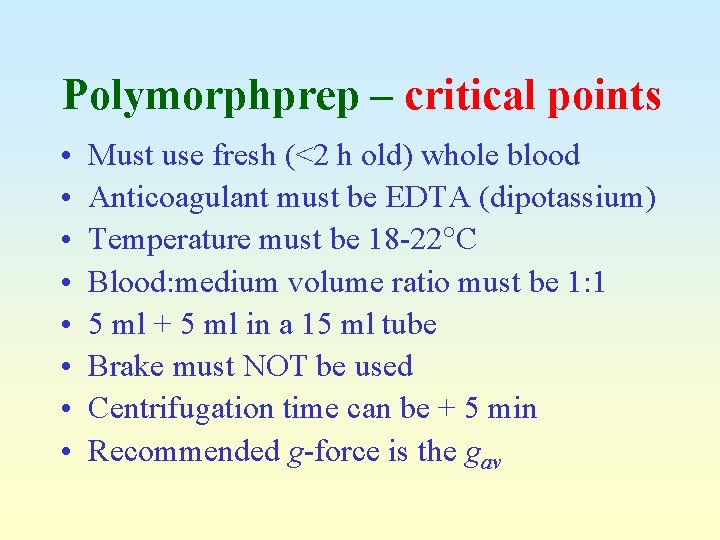
Polymorphprep – critical points • • Must use fresh (<2 h old) whole blood Anticoagulant must be EDTA (dipotassium) Temperature must be 18 -22 C Blood: medium volume ratio must be 1: 1 5 ml + 5 ml in a 15 ml tube Brake must NOT be used Centrifugation time can be + 5 min Recommended g-force is the gav

Geometry of rotors rmax rav rmin axis of rotation Set rpm speed to give 500 g at rmax Equivalent to 400 g at rav
Polymorphprep/sample volumes
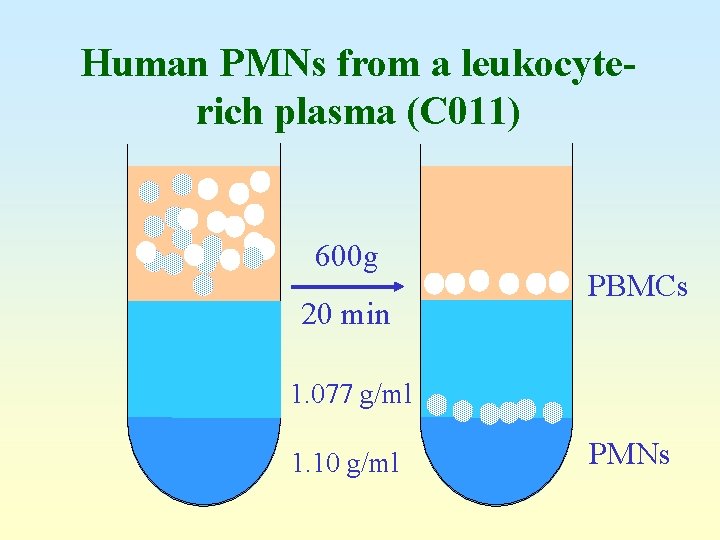
Human PMNs from a leukocyterich plasma (C 011) 600 g 20 min PBMCs 1. 077 g/ml 1. 10 g/ml PMNs
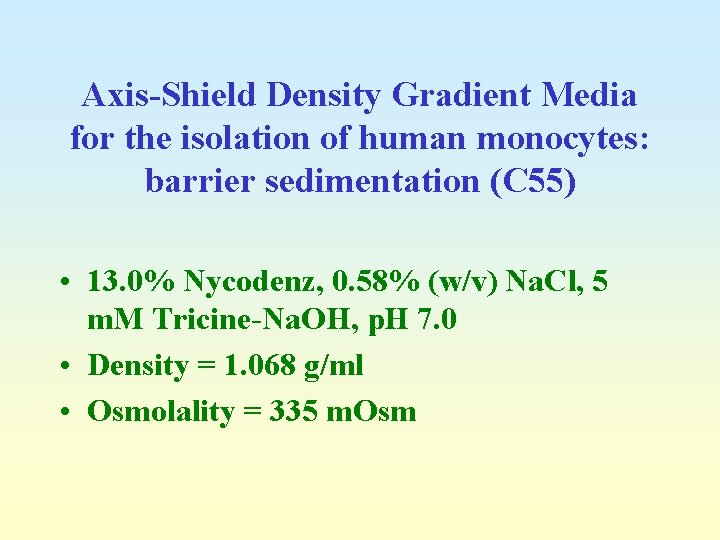
Axis-Shield Density Gradient Media for the isolation of human monocytes: barrier sedimentation (C 55) • 13. 0% Nycodenz, 0. 58% (w/v) Na. Cl, 5 m. M Tricine-Na. OH, p. H 7. 0 • Density = 1. 068 g/ml • Osmolality = 335 m. Osm
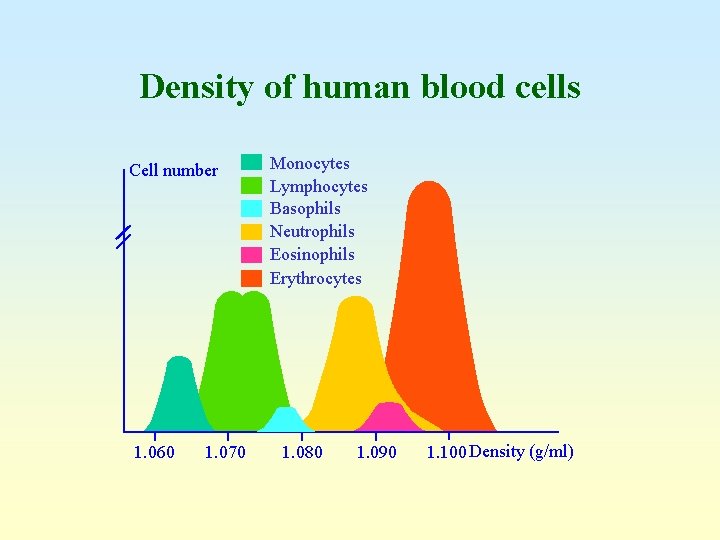
Density of human blood cells Cell number 1. 060 1. 070 Monocytes Lymphocytes Basophils Neutrophils Eosinophils Erythrocytes 1. 080 1. 090 1. 100 Density (g/ml)
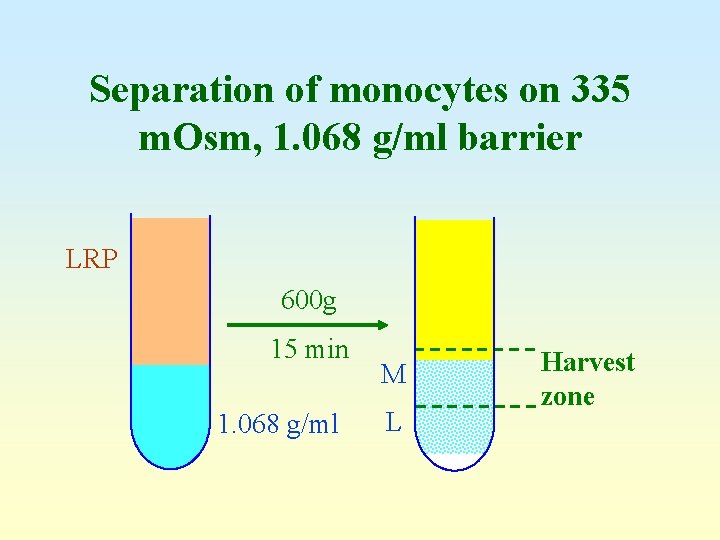
Separation of monocytes on 335 m. Osm, 1. 068 g/ml barrier LRP 600 g 15 min 1. 068 g/ml M L Harvest zone

Nycoprep™ 1. 068 requirements • • • Leukocyte-rich plasma; not whole blood Anticoagulant: EDTA Blood from normal individual, < 2 h old Room temperature 18 -22°C No brake to decelerate rotor Centrifugation conditions may need optimizing from lab to lab
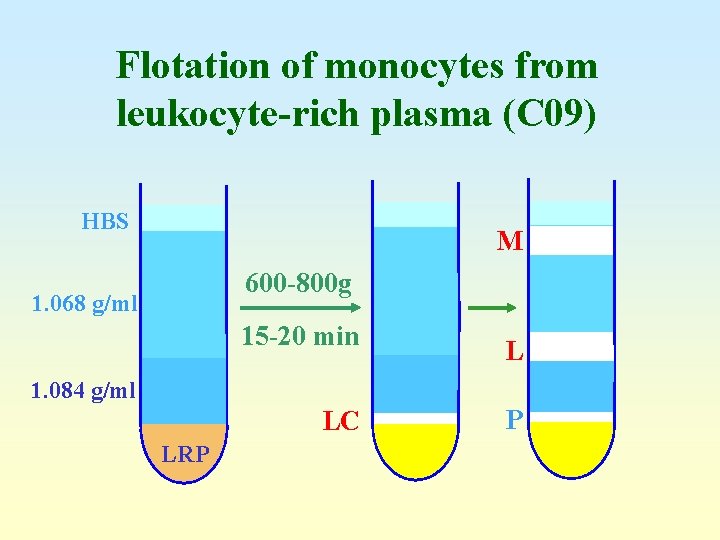
Flotation of monocytes from leukocyte-rich plasma (C 09) HBS M 600 -800 g 1. 068 g/ml 15 -20 min L 1. 084 g/ml LC LRP P

FACS analysis of monocyte band Graziani-Bowering, G. M. et al (1997)J. Immunol. Meth. , 207, 429 -436 Surface Marker % events CD 3+ 3. 4 CD 14+/CD 4– 1. 6 CD 14–/CD 4+ 6. 9 CD 14+/CD 4+ 84. 1 All monocyte markers 92. 6

Monocyte flotation isolation queries • • No monocyte band observed Band may be quite diffuse Poor recovery and purity of monocytes Rapid preparation of leukocytes essential and handling of cells must be very gentle • Does method work at 4°C? • Probably
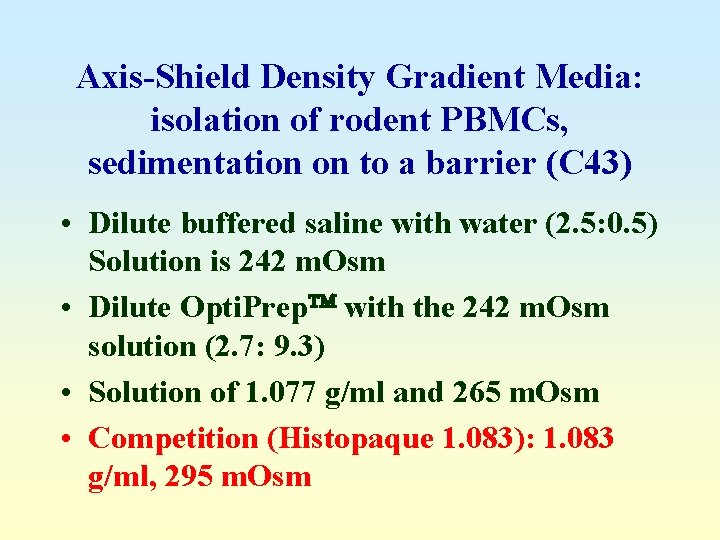
Axis-Shield Density Gradient Media: isolation of rodent PBMCs, sedimentation on to a barrier (C 43) • Dilute buffered saline with water (2. 5: 0. 5) Solution is 242 m. Osm • Dilute Opti. Prep with the 242 m. Osm solution (2. 7: 9. 3) • Solution of 1. 077 g/ml and 265 m. Osm • Competition (Histopaque 1. 083): 1. 083 g/ml, 295 m. Osm
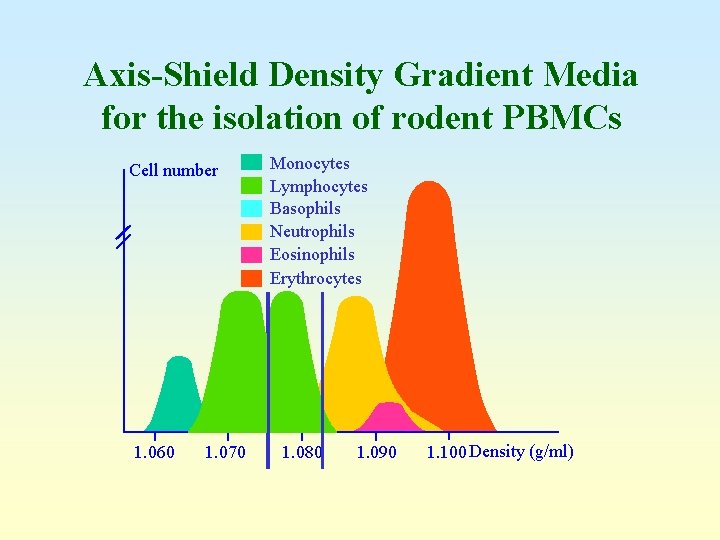
Axis-Shield Density Gradient Media for the isolation of rodent PBMCs Cell number 1. 060 1. 070 Monocytes Lymphocytes Basophils Neutrophils Eosinophils Erythrocytes 1. 080 1. 090 1. 100 Density (g/ml)
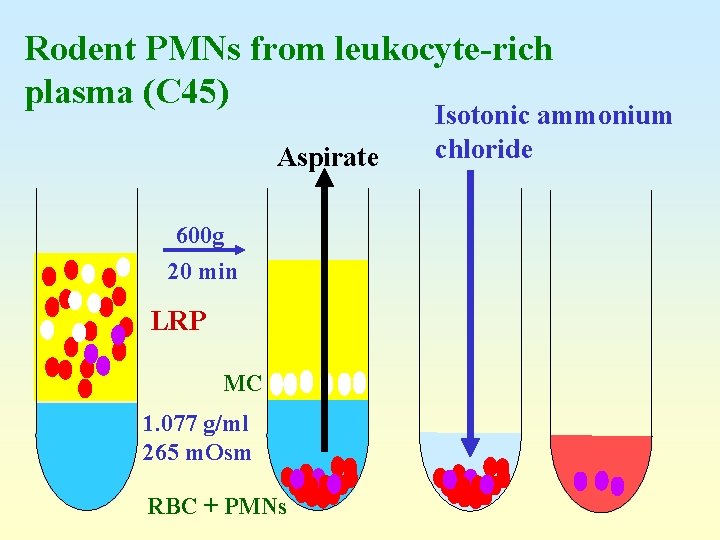
Rodent PMNs from leukocyte-rich plasma (C 45) Aspirate 600 g 20 min LRP MC 1. 077 g/ml 265 m. Osm RBC + PMNs Isotonic ammonium chloride
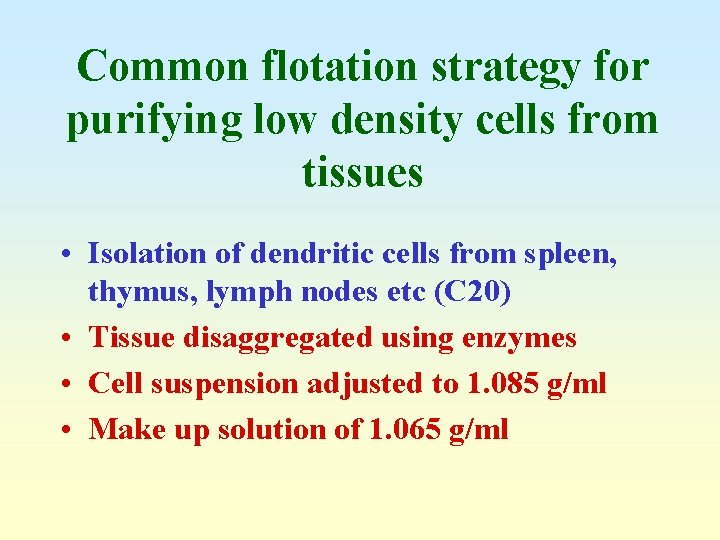
Common flotation strategy for purifying low density cells from tissues • Isolation of dendritic cells from spleen, thymus, lymph nodes etc (C 20) • Tissue disaggregated using enzymes • Cell suspension adjusted to 1. 085 g/ml • Make up solution of 1. 065 g/ml
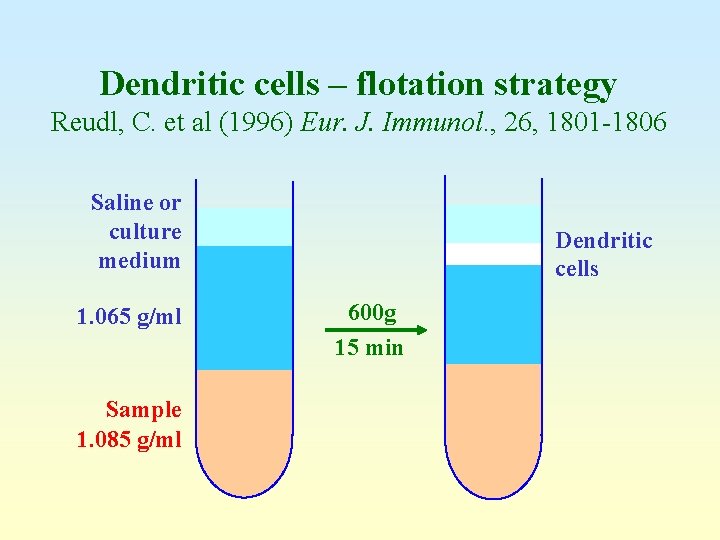
Dendritic cells – flotation strategy Reudl, C. et al (1996) Eur. J. Immunol. , 26, 1801 -1806 Saline or culture medium 1. 065 g/ml Sample 1. 085 g/ml Dendritic cells 600 g 15 min
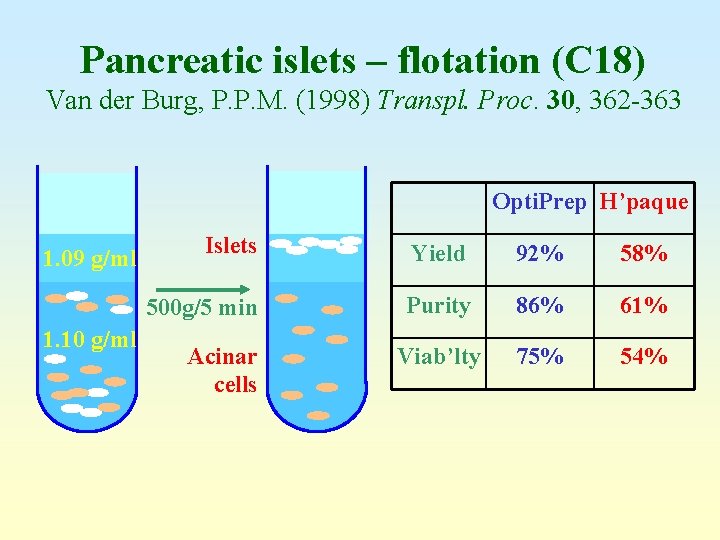
Pancreatic islets – flotation (C 18) Van der Burg, P. P. M. (1998) Transpl. Proc. 30, 362 -363 Opti. Prep H’paque 1. 09 g/ml 1. 10 g/ml Islets Yield 92% 58% 500 g/5 min Purity 86% 61% Viab’lty 75% 54% Acinar cells
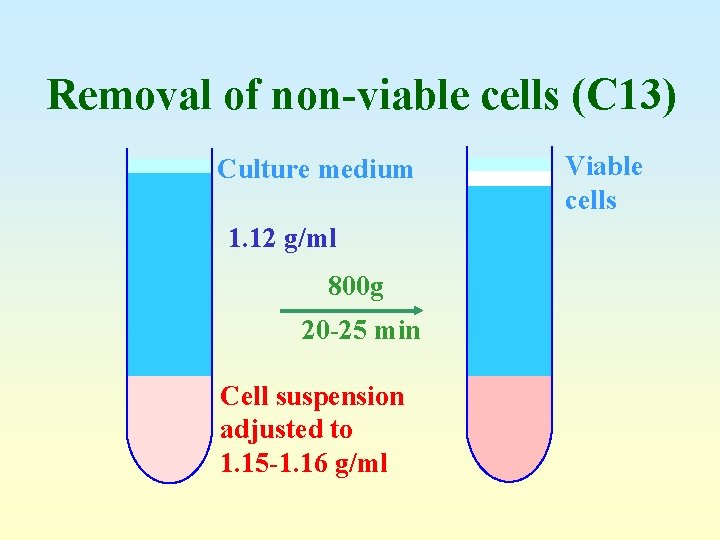
Removal of non-viable cells (C 13) Culture medium 1. 12 g/ml 800 g 20 -25 min Cell suspension adjusted to 1. 15 -1. 16 g/ml Viable cells

Other Axis-Shield Cell Applications • • Epithelial cells from gastric mucosa (C 28) Neurons: spinal cord (C 22) brain (C 29) Human erythrocytes and reticulocytes (C 35) Megakaryocytic progenitor cells (C 23/C 48) Pneumocytes and other lung cells (C 25/C 44) Stellate cells from liver and pancreas (C 33) Hepatic Kupffer cells (C 50) Renal cells (C 42)
Publications database on cells • • • Opti. Prep (since 1994) over 650 Nycodenz® (since 1984) approx 2000 Using either the Applications CD or the website: www. axis-shield-density-gradient-media. com Follow the instructions to access the relevant Index • Click on the cell of interest
- Slides: 39