Pure Substances Mixtures Solutions Power Point for week
Pure Substances, Mixtures, Solutions Power. Point for week of 9/25/15
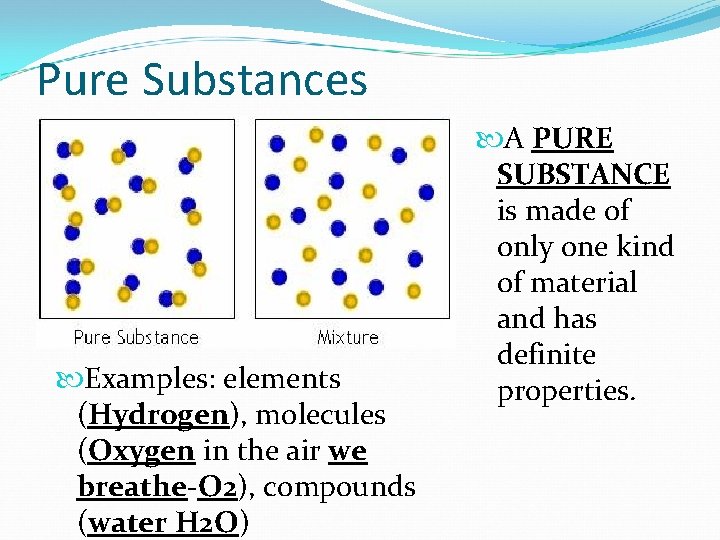
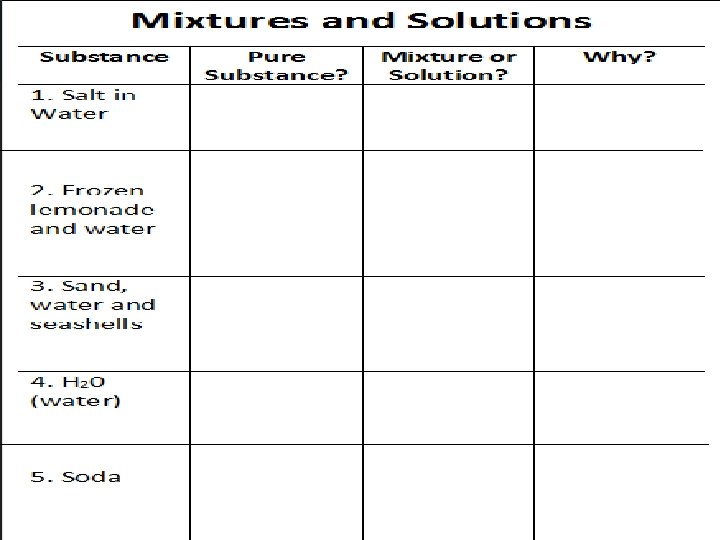
Pure Substances Examples: elements (Hydrogen), molecules (Oxygen in the air we breathe-O 2), compounds (water H 2 O) A PURE SUBSTANCE is made of only one kind of material and has definite properties.
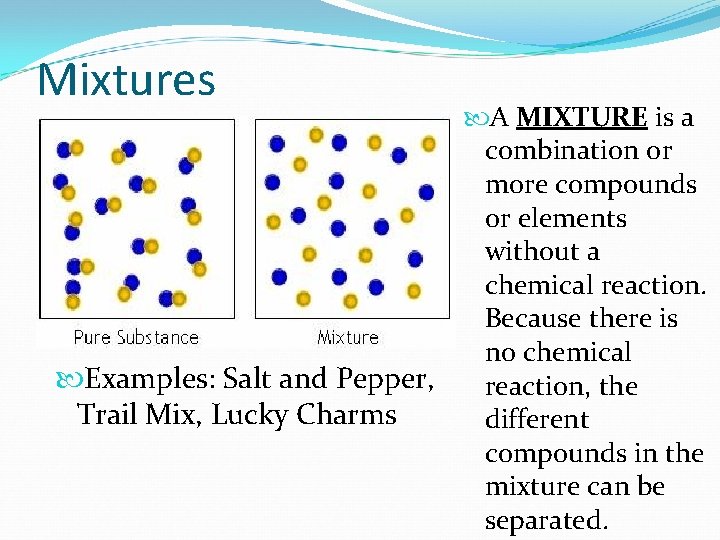
Mixtures Examples: Salt and Pepper, Trail Mix, Lucky Charms A MIXTURE is a combination or more compounds or elements without a chemical reaction. Because there is no chemical reaction, the different compounds in the mixture can be separated.
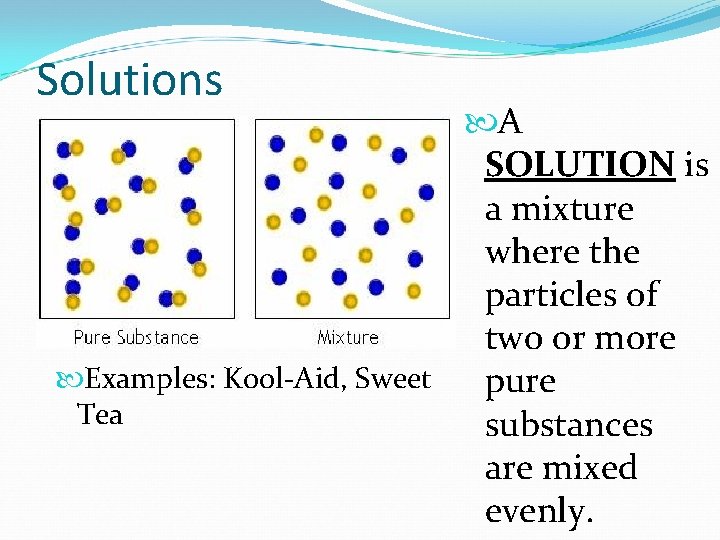
Solutions A SOLUTION is a mixture where the particles of two or more Examples: Kool-Aid, Sweet pure Tea substances are mixed evenly.
Coloring the Periodic Table Families Some images are from www. chem 4 kids. com www. middleschoolscience. com 2008
Families on the Periodic Table Elements on the periodic table can be grouped into families based on their chemical properties. Each family has a specific name to differentiate it from the other families in the periodic table. Elements in each family react differently with other elements.
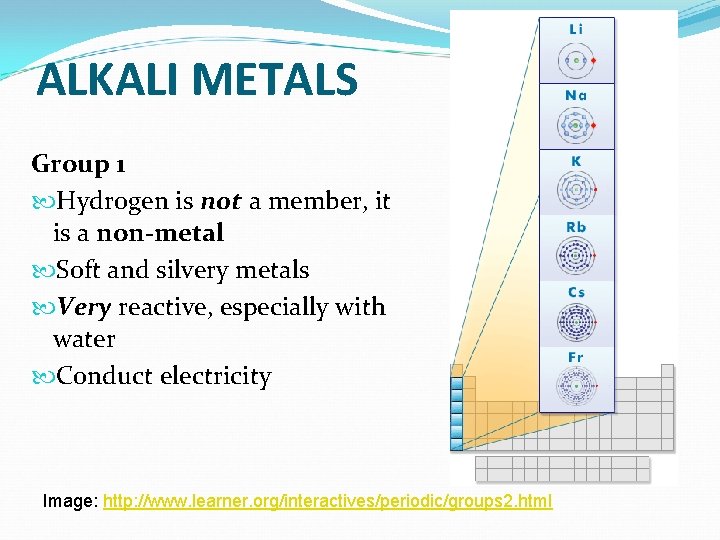
ALKALI METALS Group 1 Hydrogen is not a member, it is a non-metal Soft and silvery metals Very reactive, especially with water Conduct electricity Image: http: //www. learner. org/interactives/periodic/groups 2. html
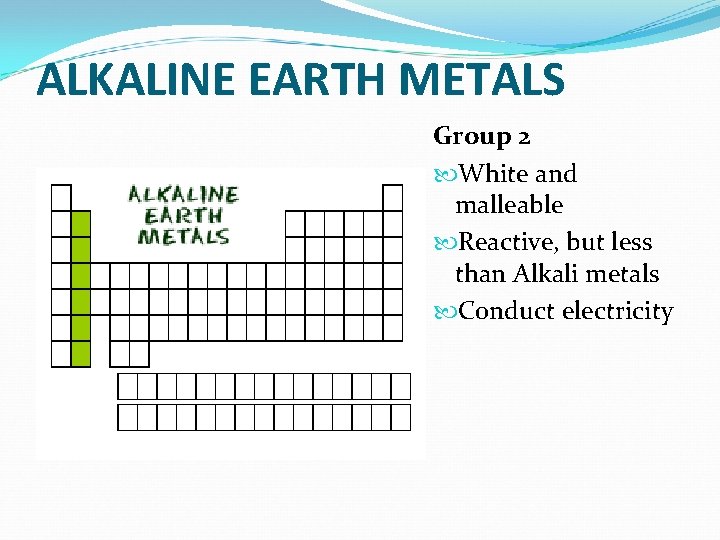
ALKALINE EARTH METALS Group 2 White and malleable Reactive, but less than Alkali metals Conduct electricity
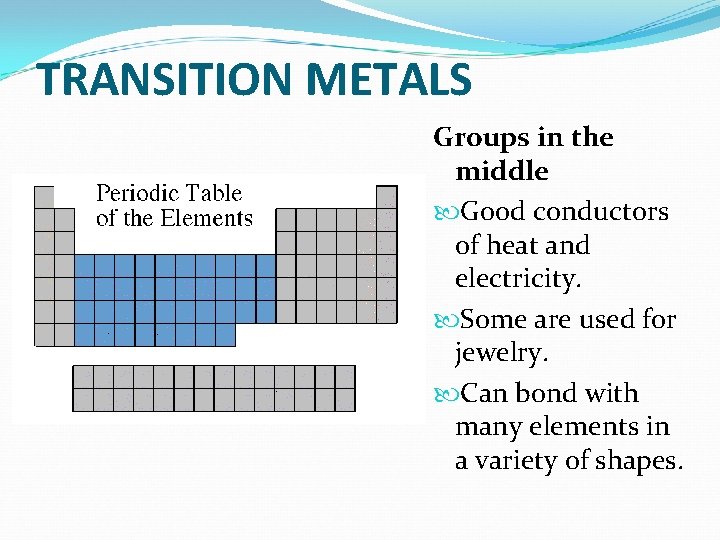
TRANSITION METALS Groups in the middle Good conductors of heat and electricity. Some are used for jewelry. Can bond with many elements in a variety of shapes.
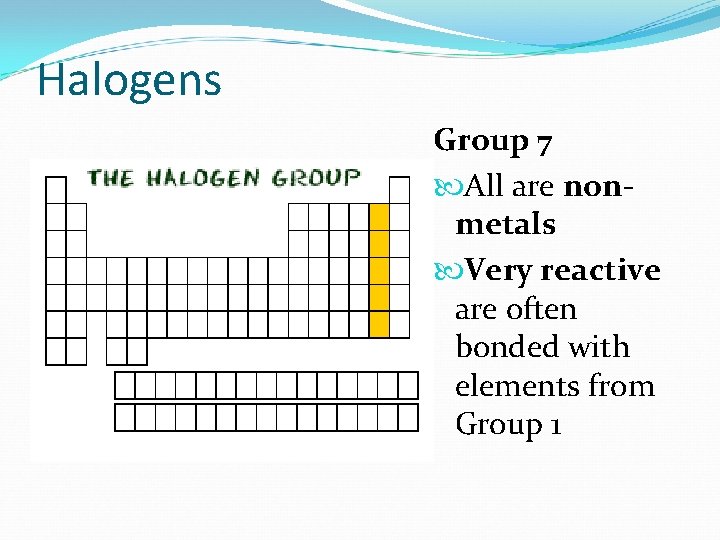
Halogens Group 7 All are nonmetals Very reactive are often bonded with elements from Group 1
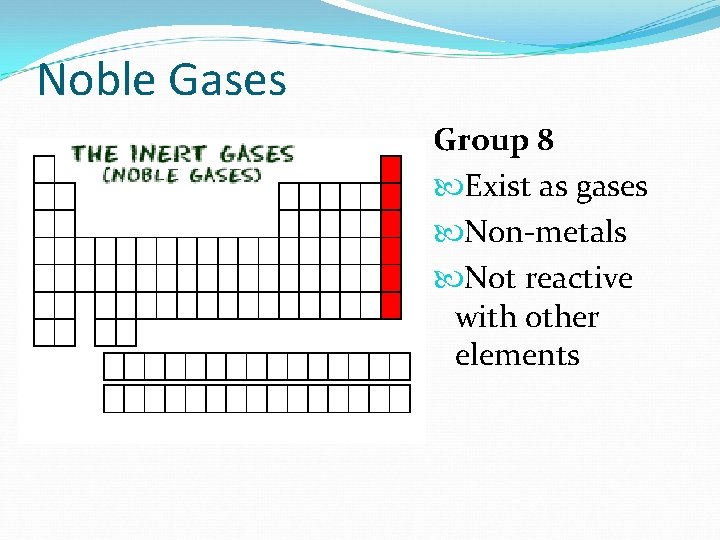
Noble Gases Group 8 Exist as gases Non-metals Not reactive with other elements
Rare Earth Metals Some are Radioactive The rare earths are silver, silverywhite, or gray metals. Conduct electricity
PERIODIC TABLE QUIZ 9/25/15

QUIZ 1) HOW ARE ELEMENTS GROUPED INTO FAMILIES ON THE PERIODIC TABLE? A. BASED ON THEIR CHEMICAL PROPERTIES B. WHAT THEY LOOK LIKE C. HOW MUCH THEY WEIGH D. BASED ON THEIR STATE OF MATTER

QUIZ 2) WHICH OF THE FOLLOWING IS NOT A MEMBER OF GROUP 1? A. LITHIUM B. SODIUM C. HYDROGEN D. POTASSIUM

QUIZ 3) GROUP 1 HAS A LOVE-HATE RELATIONSHIP WITH WHICH GROUP? A. GROUP 2 B. TRANSITION METALS C. GROUP 8 D. GROUP 7

QUIZ 4) WHAT IS THE NAME OF GROUP 2? A. ALKALI METALS B. ALKALINE EARTH METALS C. TRANSITION METALS D. METALLOIDS

QUIZ 5) WHERE ARE THE TRANSITION METALS LOCATED? A. ON THE FAR LEFT B. ON THE FAR RIGHT C. IN THE MIDDLE D. AT THE VERY BOTTOM

QUIZ 6) ARE ALL HALOGENS (GROUP 7) NON-METALS? A. YES B. NO

QUIZ 7) ELEMENTS IN GROUP 8 ARE CALLED? A. ALKALI METALS B. ALKALINE EARTH METALS C. HALOGENS D. NOBLE GASES

QUIZ 8) NOBLE GASES ARE… A. VERY REACTIVE B. VERY STABLE C. METALS D. TRANSITION METALS

QUIZ 9) SOME RARE EARTH METALS ARE: A. NON-METALS B. RADIOACTIVE C. GASES D. POOR METALS

QUIZ 10) WHICH ELEMENTS ARE COMMONLY REFERRED TO AS THE “STAIR-STEP” ELEMENTS? A. TRANSITION METALS B. HALOGENS C. NOBLE GASES D. METALLOIDS
- Slides: 24