Pure Substances Elements vs Compounds Todays Focus Recall

Pure Substances Elements vs. Compounds
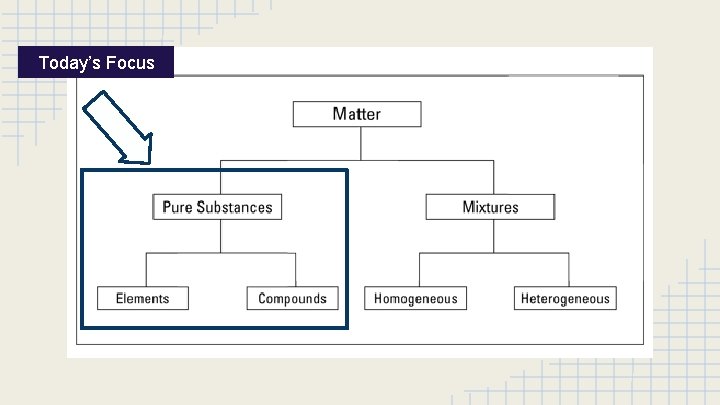
Today’s Focus
Recall Matter Physical substance Occupies space Properties Mass Volume Density ● 3 states of matter ○ Solid ○ Liquid ○ Gas Atoms & Molecules Atoms Extremely small Made of: electrons (-), protons (+), and neutrons Molecules Made up of atoms Two or more atoms Ex. H 2 O, O 2, CO 2

What makes a substance “Pure”? Homogenous Uniform composition throughout MANY identical particles (elements or compounds) ● Properties are constant throughout the whole mixture i. e When you divide water in half both halves are identical Constant chemical composition i. e When you divide water in half it will be H 2 O in both halves NOT H 2 in one half and O in the other
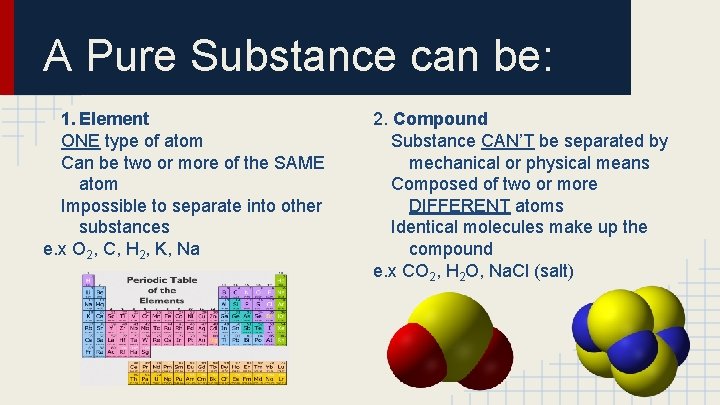
A Pure Substance can be: 1. Element ONE type of atom Can be two or more of the SAME atom Impossible to separate into other substances e. x O 2, C, H 2, K, Na 2. Compound Substance CAN’T be separated by mechanical or physical means Composed of two or more DIFFERENT atoms Identical molecules make up the compound e. x CO 2, H 2 O, Na. Cl (salt)
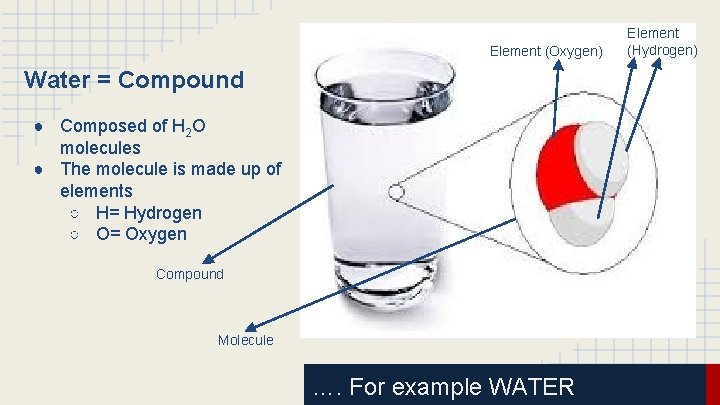
Element (Oxygen) Water = Compound ● Composed of H 2 O molecules ● The molecule is made up of elements ○ H= Hydrogen ○ O= Oxygen Compound Molecule …. For example WATER Element (Hydrogen)
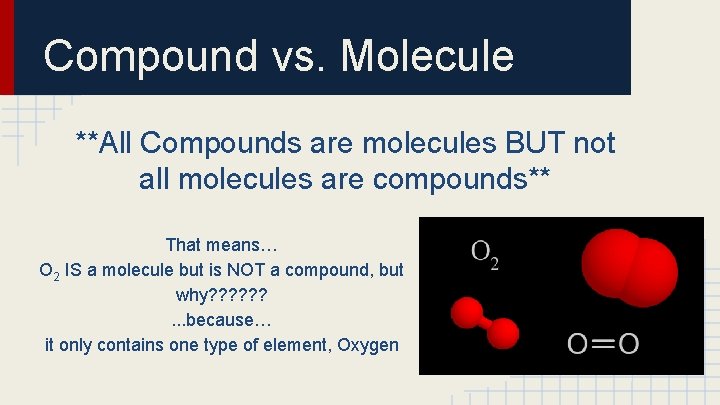
Compound vs. Molecule **All Compounds are molecules BUT not all molecules are compounds** That means… O 2 IS a molecule but is NOT a compound, but why? ? ? . . . because… it only contains one type of element, Oxygen

Take Away! Pure Substances Made of many identical molecules Smallest particle of matter
- Slides: 8