Pure Substances and Mixtures Be Careful Mixture Homogeneous
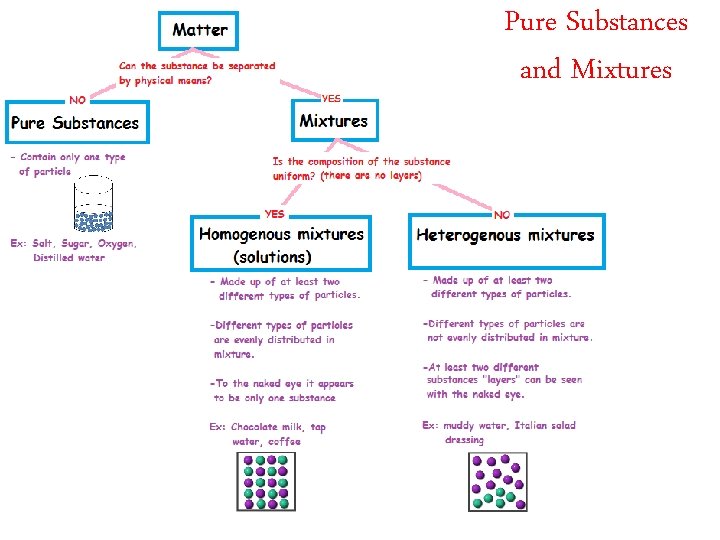
Pure Substances and Mixtures
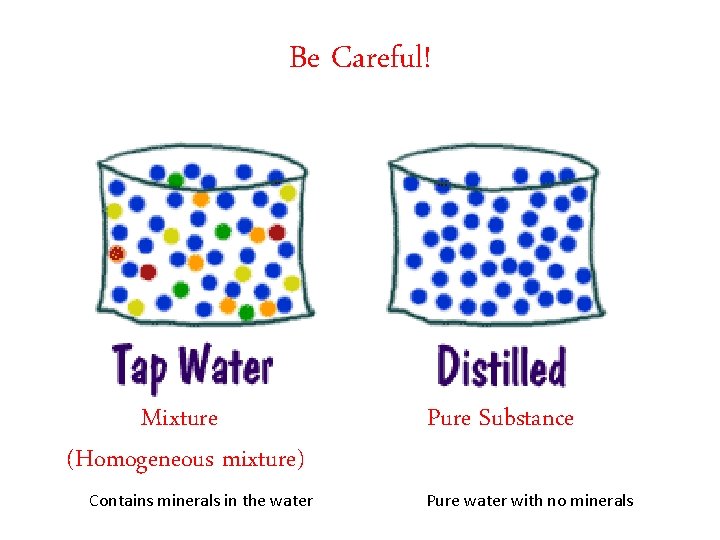
Be Careful! Mixture (Homogeneous mixture) Contains minerals in the water Pure Substance Pure water with no minerals

Homogenous Mixtures àContain two or more substances mixed together àThe particles of each substance are evenly distributed in the mixture. à 1 phase (no layers) àAlso called solutions Ex: Air, tap water, milk, apple juice Out of the two substances present: - The one present in the larger quantity is called the SOLVENT (the stuff that does the dissolving) - The one present in the smaller quantity is called the SOLUTE (the stuff being dissolved)
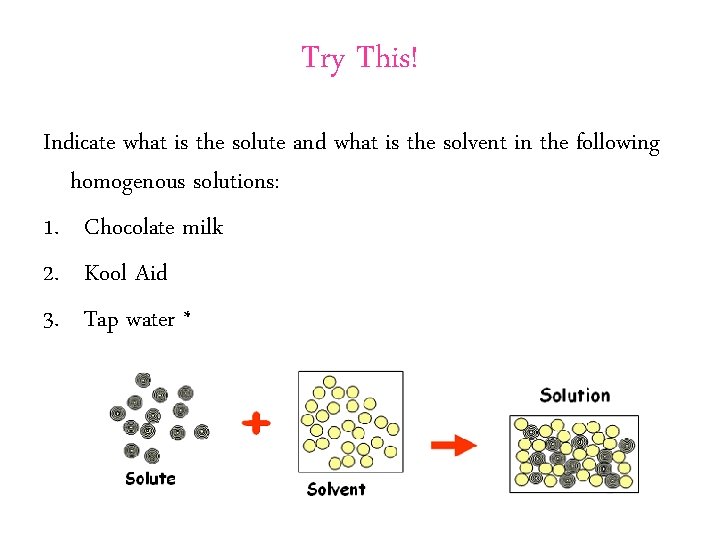
Try This! Indicate what is the solute and what is the solvent in the following homogenous solutions: 1. Chocolate milk 2. Kool Aid 3. Tap water *

Try This! Indicate whether each statement below describes a homogeneous or heterogeneous mixture: 1. Only displays one phase to the naked eye. 2. Displays an arrangement of particles that is not uniform. 3. Displays more than one phase to the naked eye or through a microscope. 4. Shows a uniform distribution of particles. 5. 6. 7. Noodle Soup Vinegar Milk

Note: In a homogenous solution, there is only one solvent (the substance in largest quantity… the stuff that does the dissolving). However, there can be more than one solute! Ex: The air we breathe is an example of a homogenous mixture. It is composed of 78% nitrogen, 21% oxygen, 0. 01% carbon dioxide and 1% other gases. Name the solvent and solutes.
- Slides: 6