Pulsed Glow Discharge Mass Spectrometry An Ionization Source


- Slides: 22

Pulsed Glow Discharge Mass Spectrometry: An Ionization Source for Aerosol Analysis and Laser Sampling Farzad Fani-Pakdel Qualifying Examination University of Florida - Department of Chemistry Division of Analytical Chemistry

Outline Introduction Aerosols characterization Glow discharge Research objective Experimental Instrumental set-up Particle introduction into system Preliminary Results Laser Sampling Importance and application Cathode design

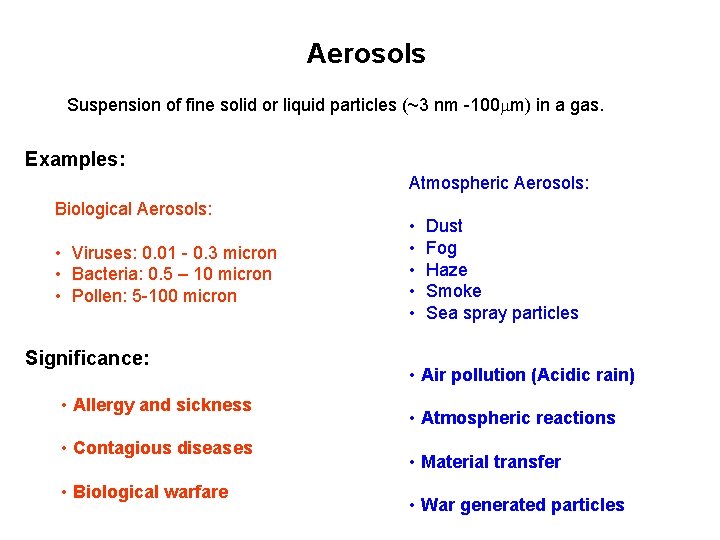
Aerosols Suspension of fine solid or liquid particles (~3 nm -100 mm) in a gas. Examples: Atmospheric Aerosols: Biological Aerosols: • Viruses: 0. 01 - 0. 3 micron • Bacteria: 0. 5 – 10 micron • Pollen: 5 -100 micron Significance: • Allergy and sickness • Contagious diseases • Biological warfare • • • Dust Fog Haze Smoke Sea spray particles • Air pollution (Acidic rain) • Atmospheric reactions • Material transfer • War generated particles

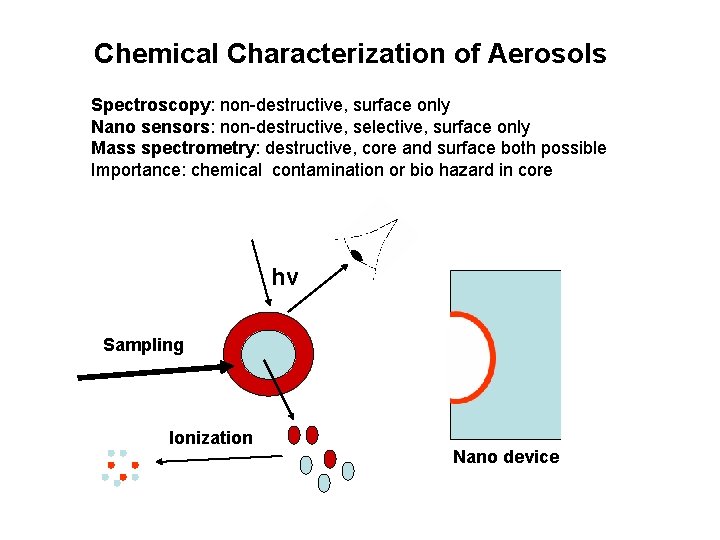
Chemical Characterization of Aerosols Spectroscopy: non-destructive, surface only Nano sensors: non-destructive, selective, surface only Mass spectrometry: destructive, core and surface both possible Importance: chemical contamination or bio hazard in core hv Sampling Ionization Nano device

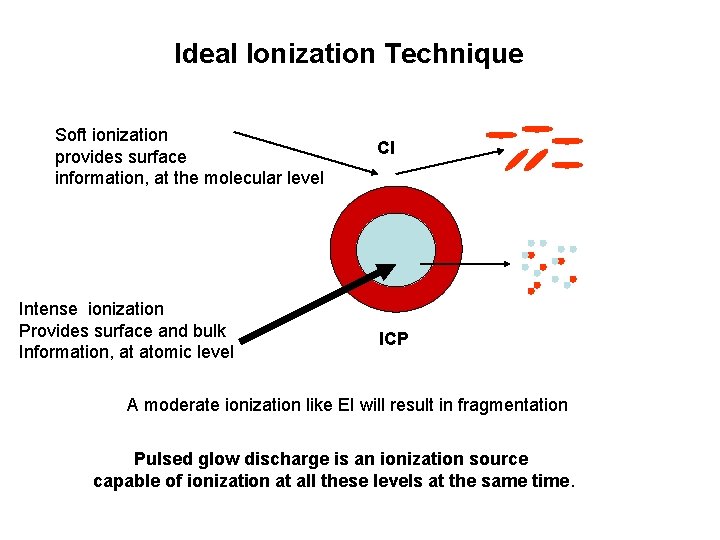
Ideal Ionization Technique Soft ionization provides surface information, at the molecular level Intense ionization Provides surface and bulk Information, at atomic level CI ICP A moderate ionization like EI will result in fragmentation Pulsed glow discharge is an ionization source capable of ionization at all these levels at the same time.

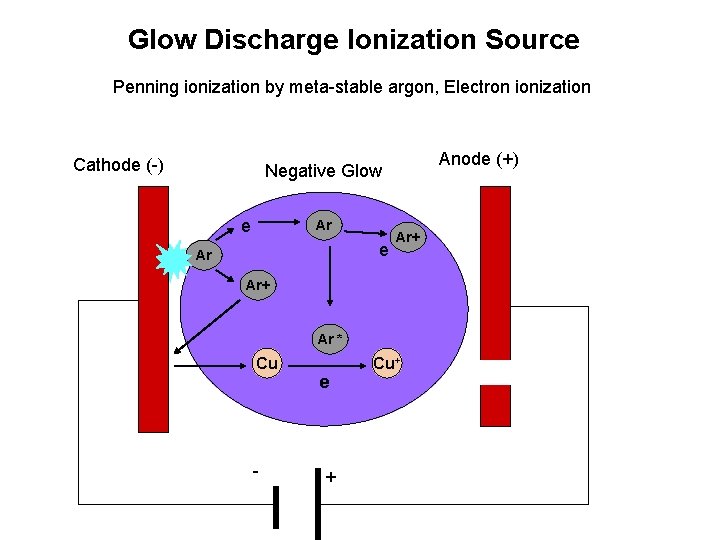
Glow Discharge Ionization Source Penning ionization by meta-stable argon, Electron ionization Cathode (-) Anode (+) Negative Glow e Ar Ar+ Ar * Cu - e + Cu+

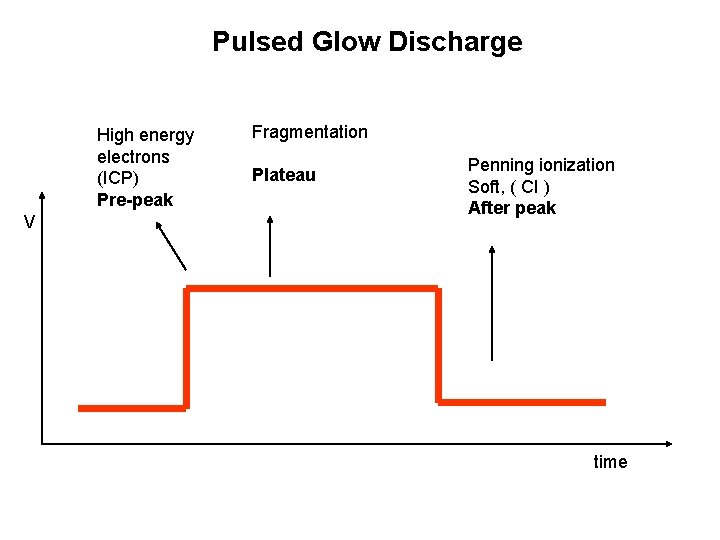
Pulsed Glow Discharge High energy electrons (ICP) Pre-peak V Fragmentation Plateau Penning ionization Soft, ( CI ) After peak time

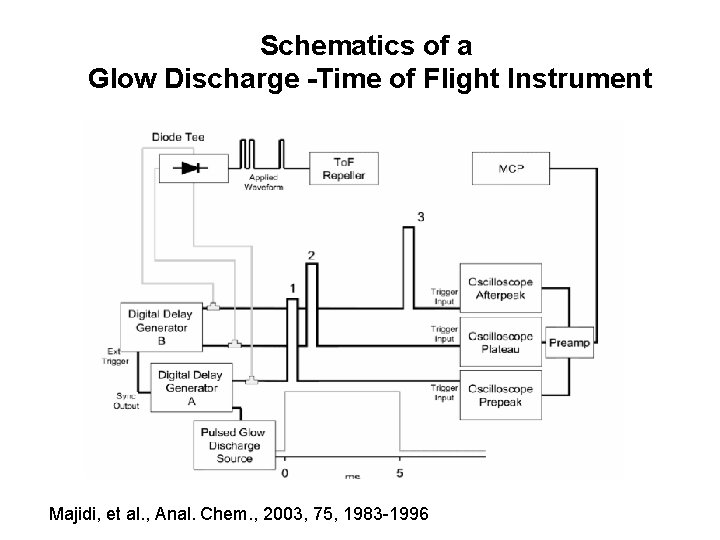
Schematics of a Glow Discharge -Time of Flight Instrument Majidi, et al. , Anal. Chem. , 2003, 75, 1983 -1996

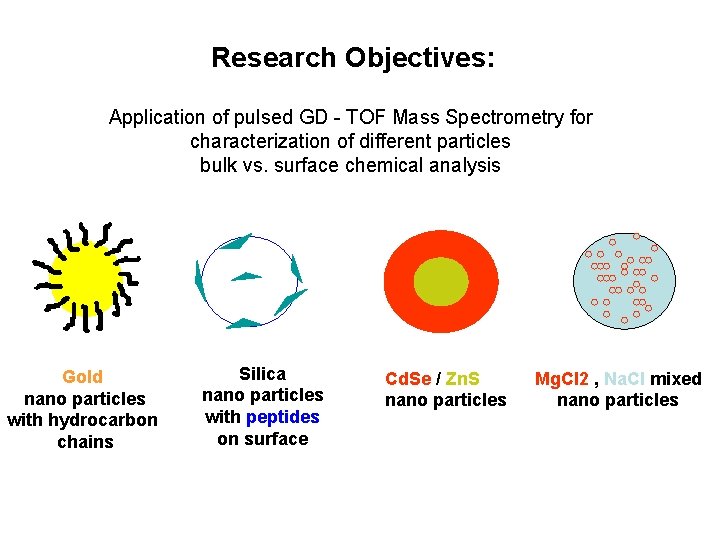
Research Objectives: Application of pulsed GD - TOF Mass Spectrometry for characterization of different particles bulk vs. surface chemical analysis Gold nano particles with hydrocarbon chains Silica nano particles with peptides on surface Cd. Se / Zn. S nano particles Mg. Cl 2 , Na. Cl mixed nano particles

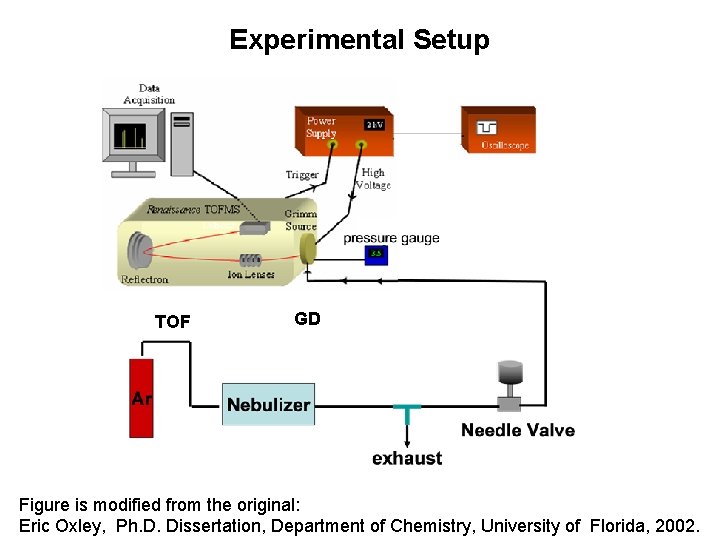
Experimental Setup TOF GD Figure is modified from the original: Eric Oxley, Ph. D. Dissertation, Department of Chemistry, University of Florida, 2002.

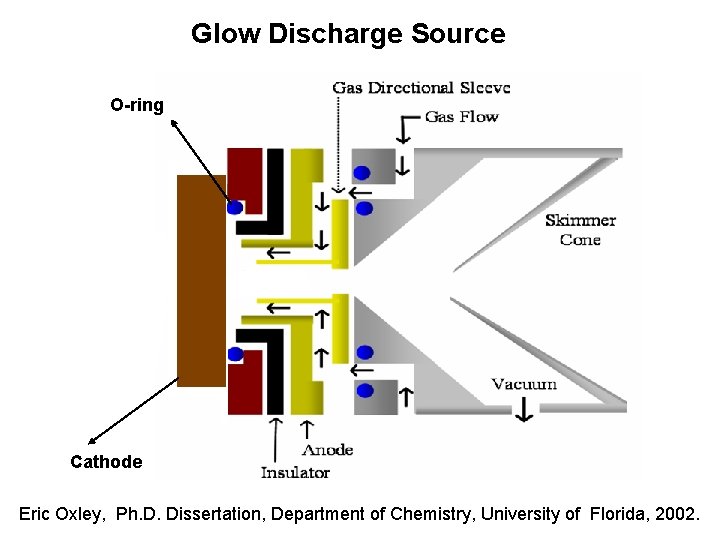
Glow Discharge Source O-ring Cathode Eric Oxley, Ph. D. Dissertation, Department of Chemistry, University of Florida, 2002.

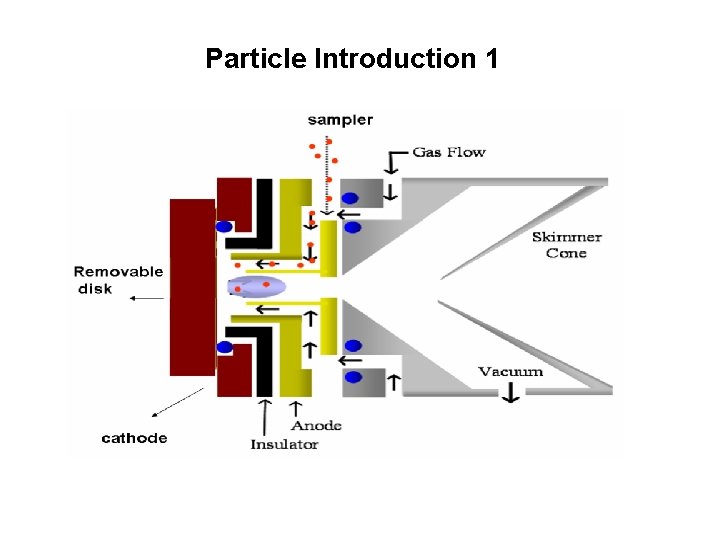
Particle Introduction 1

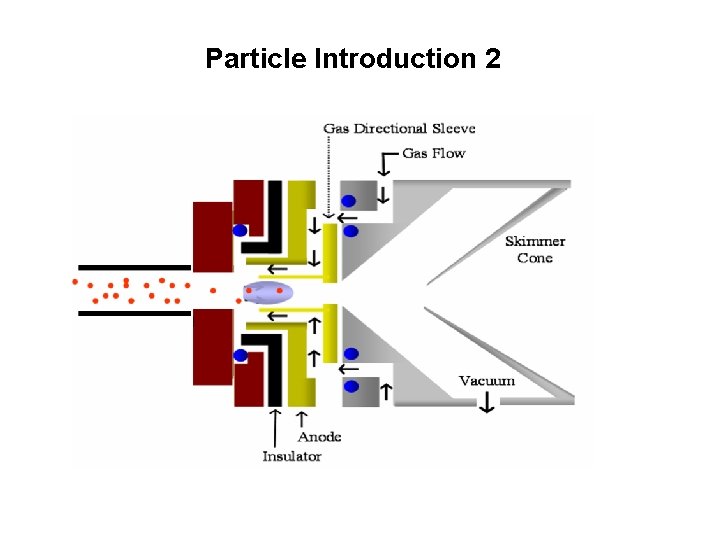
Particle Introduction 2

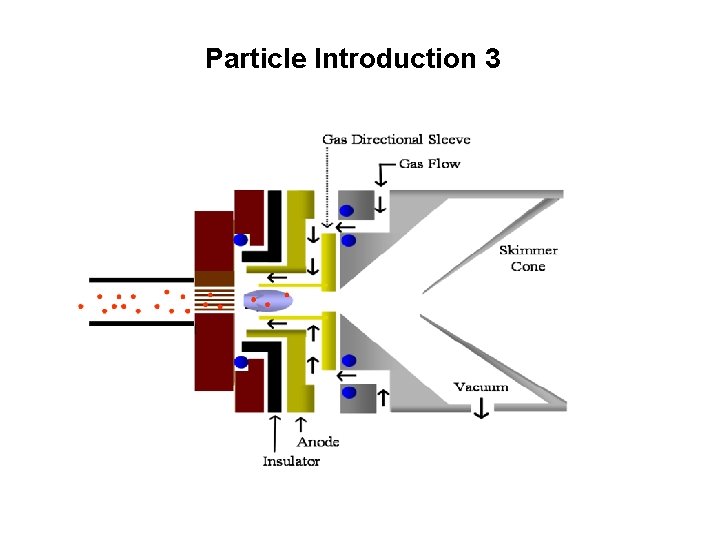
Particle Introduction 3

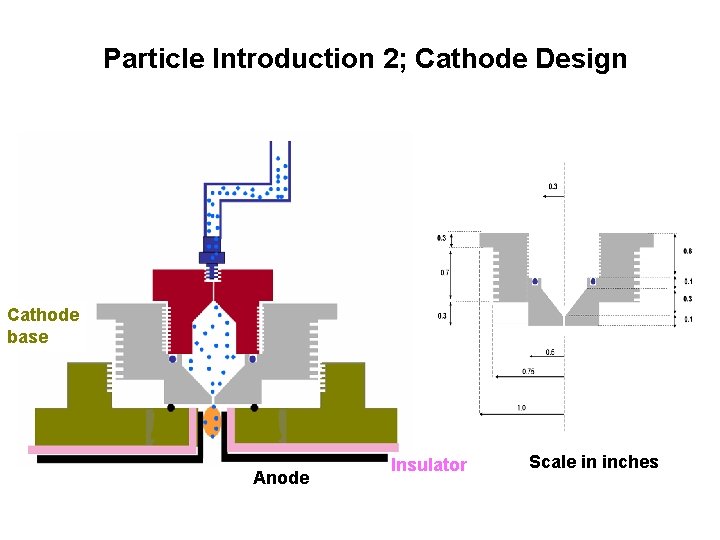
Particle Introduction 2; Cathode Design Cathode base Anode Insulator Scale in inches

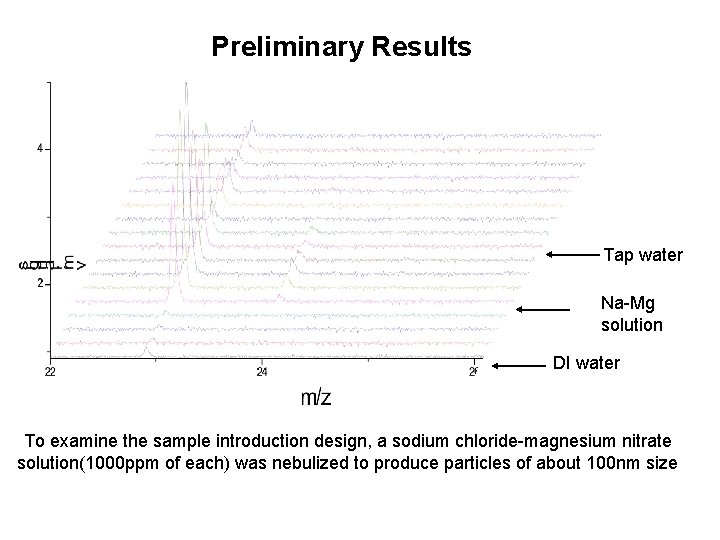
Preliminary Results Tap water Na-Mg solution DI water To examine the sample introduction design, a sodium chloride-magnesium nitrate solution(1000 ppm of each) was nebulized to produce particles of about 100 nm size

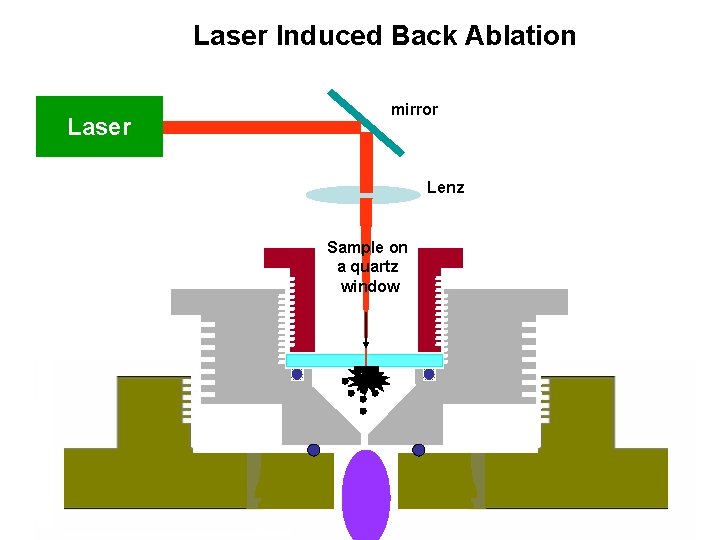
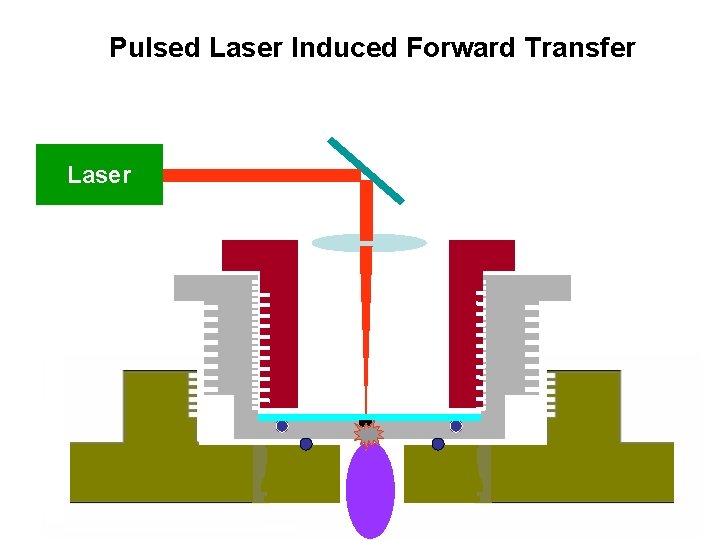
Laser Sampling: Introduction of particles /atoms into discharge by laser pulse from the sample’s back side. • Synchronized sample introduction is possible • Study of non-conductive thin films also possible, surface analysis of thin films (brain slice) • Sampling yield improves • Back side sampling (LIFT or LIBA) has the advantage of sampling directionality

Laser Induced Back Ablation Laser mirror Lenz Sample on a quartz window

Pulsed Laser Induced Forward Transfer Laser

Summary Glow discharge time of flight mass spectrometry can be used for surface and core chemical characterization of particles Cathode of the source needs to be modified for sample introduction An orifice in the cathode seems to be a proper way of particle introduction Laser sampling can be used for synchronized introduction of particles and also analysis of thin films (useful for non-conductive samples)

References: 1. Eric Oxley, “The Microsecond Pulsed Glow Discharge: Developments in Time of Flight Mass Spectrometry and Atomic Emission Spectrometry. ”, Ph. D. Dissertation, Department of Chemistry, University of Florida, 2002. W. W. Harrison, C. Yang and E. Oxley, Anal. Chem. , 2001, 73, 480 A-487 A 3. C. L. Lewis, M. A. Moser, D. E. Dale, Jr. , W. Hang, C. Hassel, F. L. King and V. Majidi, Anal. Chem. , 2003, 75, 1983 -1996 4. A. B. Bullock, P. R. Bolton, Appl. Phys. , 1999, 85, No. 1, 460 -465

Acknowledgments: • Dr Omenetto • Dr Harrison • Dr Igor Gornushkin • • • Dr Winefordner • Dr Smith • Dr Kevin Turnery Committee members Winefordner-Omenetto- Smith group Friends from Yost / Martin group Machine shop Electronic Shop