PTT 3102 WASTE MANAGEMENT AND UTILIZATION SEM 2







- Slides: 30

PTT 310/2 WASTE MANAGEMENT AND UTILIZATION SEM 2 (2013/2014) Lecture 3: Wastewater and its Characteristics

Student should be able to; EXPLAIN, DEMONSTRATE, and DISCUSS the types and characteristics of wastewater

Wastewater ? ? any water @ liquid that contains impurities or pollutants in the form of solids or gasses or their combinations in such a concentration that is harmful if disposed into the environment Impurities in ww are mainly due to the presence of solids in the water. The solids may be organic or inorganics in nature and may be present in suspended, colloidal, dissolved or in the various forms of their combinations. The prescribed limit or acceptable level of concentration of impurities or pollutants is laid down by the local authoroties such as Jabatan Alam Sekitar. The final discharge of ww will normally be either into the body of water or onto the land. The receiving bodies of water may be streams, lakes, ponds, canals, rivers, seas etc.

Domestic Wastewater • Municipal ww/ used water (sanitary ww) • Discharged from residences, institutional and public facilities • Contains organics & inorganics solids & microorganism (bacteria) • Composition depends on the source of its generation Industrial Wastewater • Generated by large & medium scale industries. • Vary in quantity & quality from industry to industry and process to process for the same industry • Majority of manufacturing industries generate a large volume of high strength ww.

4 CHARACTERISTICS OF WASTEWATER Physical � Related to the quality of water for domestic use. � Associated with the appearance of water � Eg. Color, turbidity, temperature, taste and odor. Chemical � Sometimes evidenced by their observed reactions (comparative performance of hard & soft waters in laundering) � Most often, differences are not visible. � Eg. Organic (carbohydrates, fats, oils, pesticides, surfactant, VOC), Inorganic (chlorides, heavy metals, nitrogen, phosphorus), gases (H 2 S. Methane, Sulfir) Biological � Very important in their relation to public health � Significant in modifying the physical and chemical characteristic of water � Eg. Animals, Plants, Protists, Viruses Radiological � Considered in areas where there is a possibility that the water may have come in contact with radioactive substances

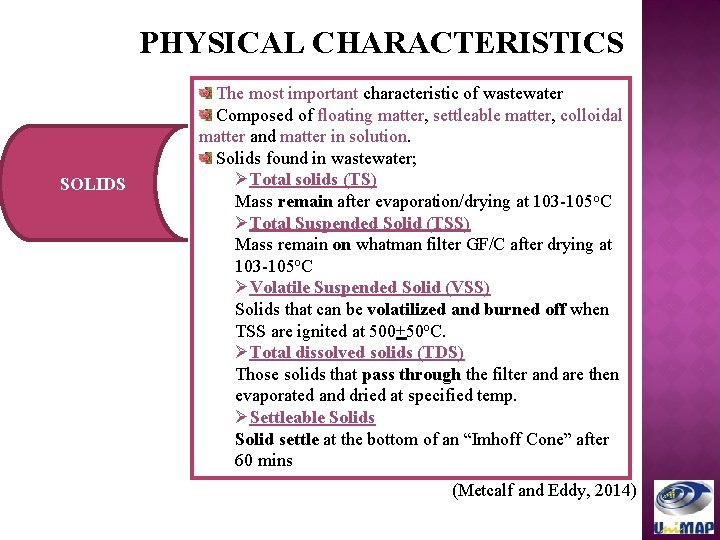
PHYSICAL CHARACTERISTICS SOLIDS The most important characteristic of wastewater Composed of floating matter, settleable matter, colloidal matter and matter in solution. Solids found in wastewater; ØTotal solids (TS) Mass remain after evaporation/drying at 103 -105 o. C ØTotal Suspended Solid (TSS) Mass remain on whatman filter GF/C after drying at 103 -105 o. C ØVolatile Suspended Solid (VSS) Solids that can be volatilized and burned off when TSS are ignited at 500+50 o. C. ØTotal dissolved solids (TDS) Those solids that pass through the filter and are then evaporated and dried at specified temp. ØSettleable Solids Solid settle at the bottom of an “Imhoff Cone” after 60 mins (Metcalf and Eddy, 2014)

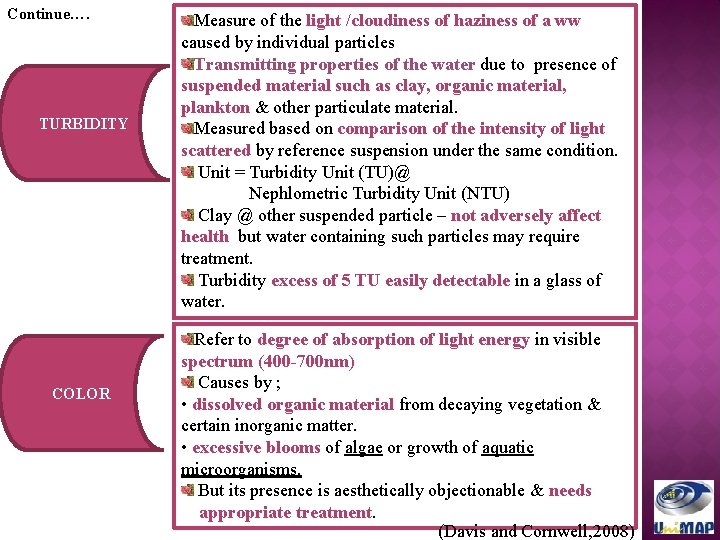
Continue…. TURBIDITY COLOR Measure of the light /cloudiness of haziness of a ww caused by individual particles Transmitting properties of the water due to presence of suspended material such as clay, organic material, plankton & other particulate material. Measured based on comparison of the intensity of light scattered by reference suspension under the same condition. Unit = Turbidity Unit (TU)@ Nephlometric Turbidity Unit (NTU) Clay @ other suspended particle – not adversely affect health but water containing such particles may require treatment. Turbidity excess of 5 TU easily detectable in a glass of water. Refer to degree of absorption of light energy in visible spectrum (400 -700 nm) Causes by ; • dissolved organic material from decaying vegetation & certain inorganic matter. • excessive blooms of algae or growth of aquatic microorganisms. But its presence is aesthetically objectionable & needs appropriate treatment. (Davis and Cornwell, 2008)

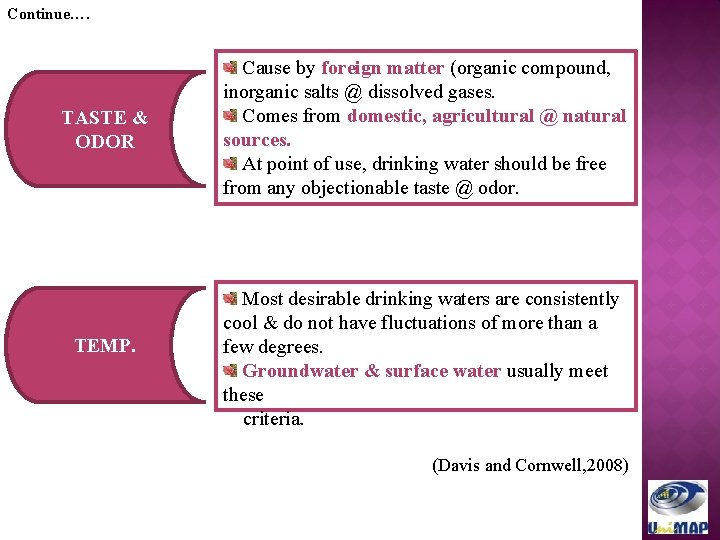
Continue…. TASTE & ODOR TEMP. Cause by foreign matter (organic compound, inorganic salts @ dissolved gases. Comes from domestic, agricultural @ natural sources. At point of use, drinking water should be free from any objectionable taste @ odor. Most desirable drinking waters are consistently cool & do not have fluctuations of more than a few degrees. Groundwater & surface water usually meet these criteria. (Davis and Cornwell, 2008)

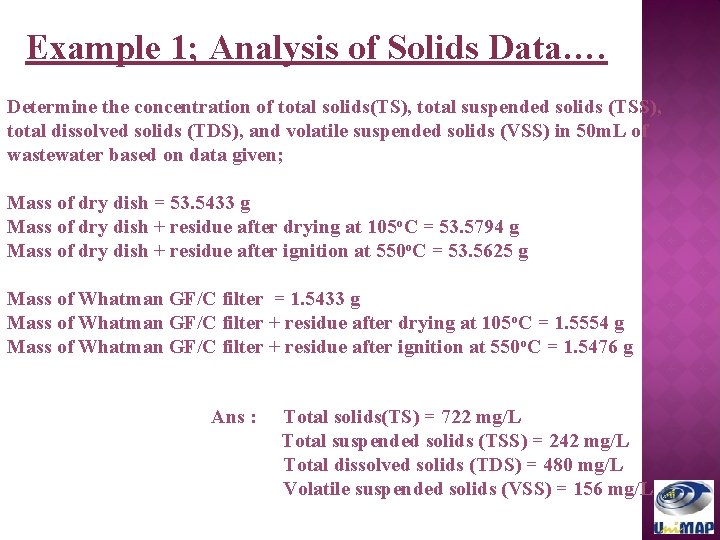
Example 1; Analysis of Solids Data…. Determine the concentration of total solids(TS), total suspended solids (TSS), total dissolved solids (TDS), and volatile suspended solids (VSS) in 50 m. L of wastewater based on data given; Mass of dry dish = 53. 5433 g Mass of dry dish + residue after drying at 105 o. C = 53. 5794 g Mass of dry dish + residue after ignition at 550 o. C = 53. 5625 g Mass of Whatman GF/C filter = 1. 5433 g Mass of Whatman GF/C filter + residue after drying at 105 o. C = 1. 5554 g Mass of Whatman GF/C filter + residue after ignition at 550 o. C = 1. 5476 g Ans : Total solids(TS) = 722 mg/L Total suspended solids (TSS) = 242 mg/L Total dissolved solids (TDS) = 480 mg/L Volatile suspended solids (VSS) = 156 mg/L

CHEMICAL CHARACTERISTICS CHLORIDE FLUORIDE Most of water contain. Amount presence causes by ; -Leaching of marine sedimentary deposits -Pollution from sea water @ brine @ industrial @ domestic waste. Chloride conc. > 250 mg/L – noticeable taste Domestic water should contain < 100 mg/L chloride. Some areas – water source contain natural fluoride. Excessive fluoride in drinking water – produce fluorosis (mottling) of teeth. Mottled – black sports @ streaks and may become brittle when exposed to large amounts of fluoride. Acceptable level for fluoride conc. between 0. 8 – 1. 3 mg/L (Davis and Cornwell, 2008)

Continue…. TOXIC INORGANIC SUBSTANCES Major clases ; a) Nitrates (NO 3) b) Cyanides (CN) c) Heavy metals constituents; – arsenics (As), barium (Ba), cadmium (Cd), chromium (Cr), lead (Pb), mercury (Hg), selenium (Se), and silver (Ag) - effects – cause poisons ( As and Cr 6+) - chronic disease (Pb, Cd, and Hg) (Davis and Cornwell, 2008) TOXIC ORGANIC SUBSTANCES There are over 120 toxic organic compounds listed on U. S. Environmental Protection Agency’s Priority Pollutant List. Eg; pesticide, insecticides and solvents. Effects may be acute @chronic. (Metcalf and Eddy, 2003)

Continue…. MEASUREMENT OF ORGANIC SUBSTANCES The analysis used to measure aggregate organic material may be divided into 2; ØTo measure gross conc. of organic substance greater than 1. 0 mg/L ØTo measure trace conc. in the range of 10 -12 to 100 mg/L Laboratory methods commonly used today to measure gross amounts of organic matter (typically greater than 1 mg/L) in wastewater include; ØBiochemical oxygen demand (BOD) ØChemical oxygen demand (COD) ØTotal organic carbon (TOC) Complementing of these laboratory tests is theoretical oxygen demand (Th. OD), which is determined from the chemical formula of the organic matter. (Metcalf and Eddy, 2003)

Continue…. Biochemical Oxygen Demand(BOD) The most widely used parameter of organic pollution 5 -day BOD – involved the measurement of the dissolved oxygen used by microorganisms in the biochemical oxidation of organic matter. BOD test results are used to; ØDetermine the appropriate quantity of oxygen that will be required to biologically stabilize the organic matter present. ØMeasure the efficiency of some treatment process ØDetermine the size of waste treatment facilities. ØDetermine compliance with wastewater discharge permits. BOD at 20 o. C for 5 days is used as standard test (measure after 5 days in incubation at 20 o. C). Use bacteria to oxidize biodegradable organic in wastewater sample after incubation. BOD can be calculates by measuring DO before & after incubation. (Metcalf and Eddy, 2003)

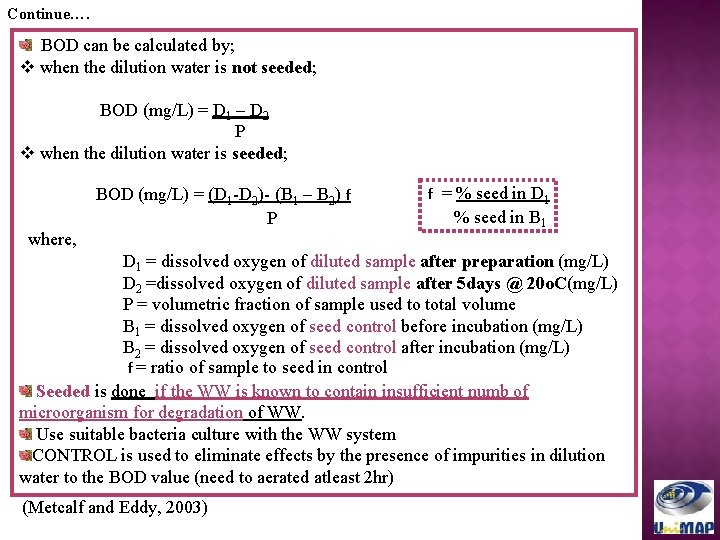
Continue…. BOD can be calculated by; v when the dilution water is not seeded; BOD (mg/L) = D 1 – D 2 P v when the dilution water is seeded; BOD (mg/L) = (D 1 -D 2)- (B 1 – B 2) f P where, f = % seed in D 1 % seed in B 1 D 1 = dissolved oxygen of diluted sample after preparation (mg/L) D 2 =dissolved oxygen of diluted sample after 5 days @ 20 o. C(mg/L) P = volumetric fraction of sample used to total volume B 1 = dissolved oxygen of seed control before incubation (mg/L) B 2 = dissolved oxygen of seed control after incubation (mg/L) f = ratio of sample to seed in control Seeded is done if the WW is known to contain insufficient numb of microorganism for degradation of WW. Use suitable bacteria culture with the WW system CONTROL is used to eliminate effects by the presence of impurities in dilution water to the BOD value (need to aerated atleast 2 hr) (Metcalf and Eddy, 2003)

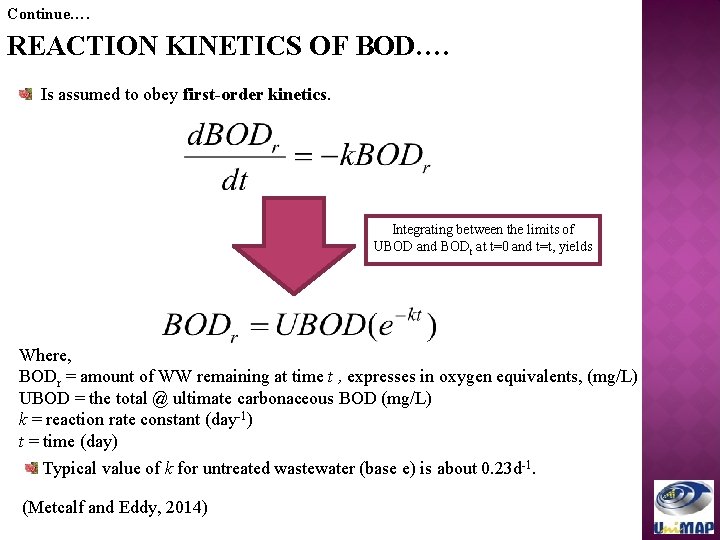
Continue…. REACTION KINETICS OF BOD…. Is assumed to obey first-order kinetics. Integrating between the limits of UBOD and BODt at t=0 and t=t, yields Where, BODr = amount of WW remaining at time t , expresses in oxygen equivalents, (mg/L) UBOD = the total @ ultimate carbonaceous BOD (mg/L) k = reaction rate constant (day-1) t = time (day) Typical value of k for untreated wastewater (base e) is about 0. 23 d-1. (Metcalf and Eddy, 2014)

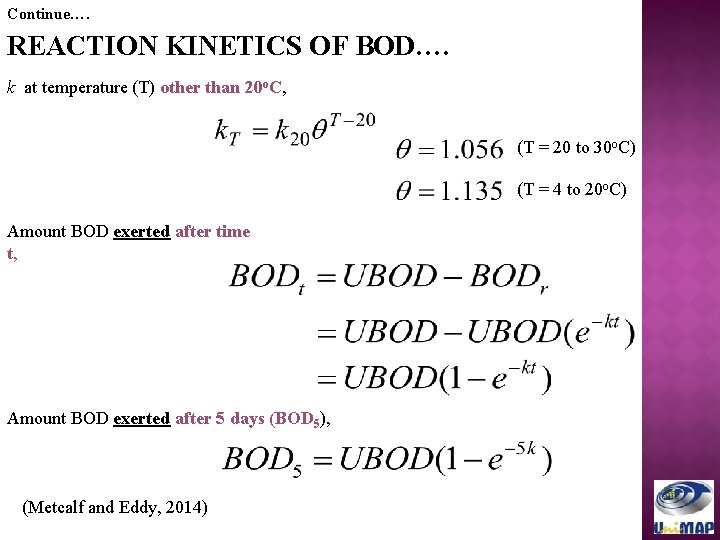
Continue…. REACTION KINETICS OF BOD…. k at temperature (T) other than 20 o. C, (T = 20 to 30 o. C) (T = 4 to 20 o. C) Amount BOD exerted after time t, Amount BOD exerted after 5 days (BOD 5), (Metcalf and Eddy, 2014)

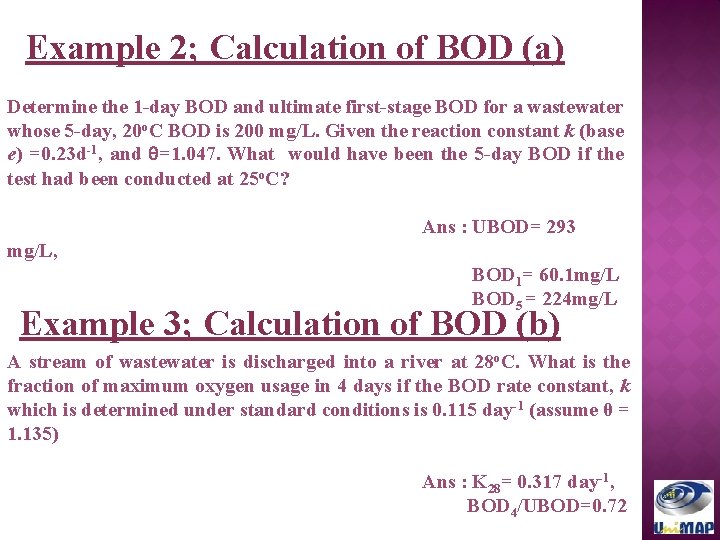
Example 2; Calculation of BOD (a) Determine the 1 -day BOD and ultimate first-stage BOD for a wastewater whose 5 -day, 20 o. C BOD is 200 mg/L. Given the reaction constant k (base e) =0. 23 d-1, and θ=1. 047. What would have been the 5 -day BOD if the test had been conducted at 25 o. C? Ans : UBOD= 293 mg/L, BOD 1= 60. 1 mg/L BOD 5 = 224 mg/L Example 3; Calculation of BOD (b) A stream of wastewater is discharged into a river at 28 o. C. What is the fraction of maximum oxygen usage in 4 days if the BOD rate constant, k which is determined under standard conditions is 0. 115 day-1 (assume θ = 1. 135) Ans : K 28= 0. 317 day-1, BOD 4/UBOD=0. 72

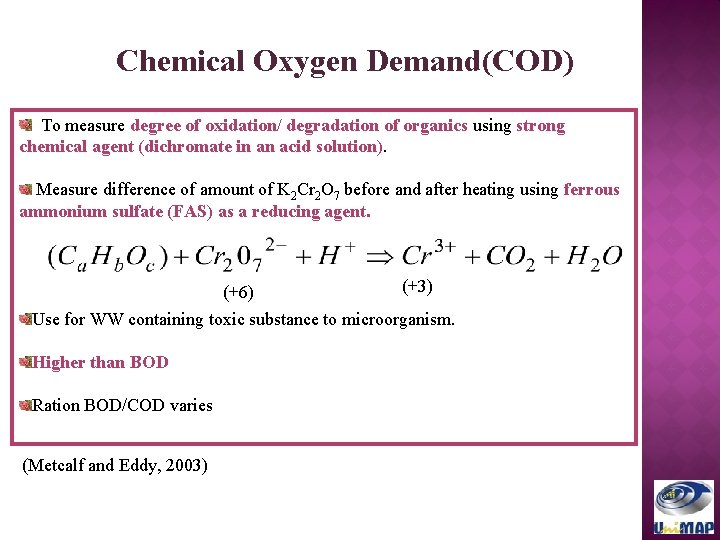
Chemical Oxygen Demand(COD) To measure degree of oxidation/ degradation of organics using strong chemical agent (dichromate in an acid solution). Measure difference of amount of K 2 Cr 2 O 7 before and after heating using ferrous ammonium sulfate (FAS) as a reducing agent. (+6) (+3) Use for WW containing toxic substance to microorganism. Higher than BOD Ration BOD/COD varies (Metcalf and Eddy, 2003)

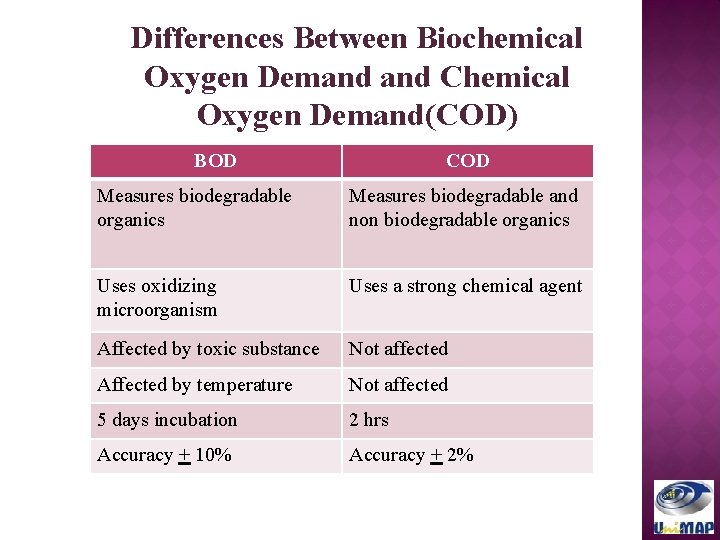
Differences Between Biochemical Oxygen Demand Chemical Oxygen Demand(COD) BOD COD Measures biodegradable organics Measures biodegradable and non biodegradable organics Uses oxidizing microorganism Uses a strong chemical agent Affected by toxic substance Not affected Affected by temperature Not affected 5 days incubation 2 hrs Accuracy + 10% (Metcalf Accuracy + 2%and Eddy, 2003)

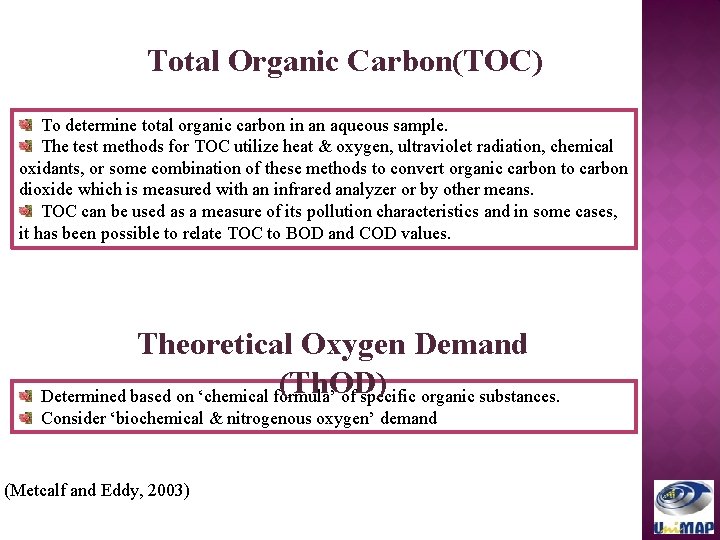
Total Organic Carbon(TOC) To determine total organic carbon in an aqueous sample. The test methods for TOC utilize heat & oxygen, ultraviolet radiation, chemical oxidants, or some combination of these methods to convert organic carbon to carbon dioxide which is measured with an infrared analyzer or by other means. TOC can be used as a measure of its pollution characteristics and in some cases, it has been possible to relate TOC to BOD and COD values. Theoretical Oxygen Demand (Th. OD) Determined based on ‘chemical formula’ of specific organic substances. Consider ‘biochemical & nitrogenous oxygen’ demand (Metcalf and Eddy, 2003)

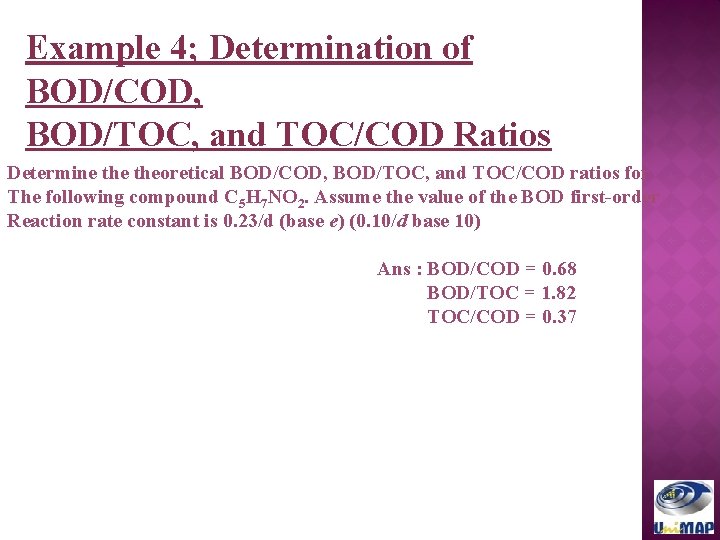
Example 4; Determination of BOD/COD, BOD/TOC, and TOC/COD Ratios Determine theoretical BOD/COD, BOD/TOC, and TOC/COD ratios for The following compound C 5 H 7 NO 2. Assume the value of the BOD first-order Reaction rate constant is 0. 23/d (base e) (0. 10/d base 10) Ans : BOD/COD = 0. 68 BOD/TOC = 1. 82 TOC/COD = 0. 37

Example 5; Calculation of Th. OD Determine the Th. OD for glycine (CH 2(NH 2)COOH) using the following assumption; a)If the 1 st step, the organic carbon & nitrogen are converted to carbon dioxide (CO 2) and ammonia (NH 3), respectively b)In the 2 nd and 3 rd steps, the ammonia is oxidized sequentially to nitrite and nitrate. c)The Th. OD is the sum of the oxygen required for all three steps. Ans : Th. OD= 112 g O 2/mol glycine.

MICROBIOLOGICAL CHARACTERISTICS DISEASE PRODUCING ORGANISMS (pathogens) Disease-producing organisms (pathogens) – viruses, bacteria, protozoa and helminths (worms). Water for drinking & cooking purposes must be made FREE from pathogens Some organism can cause disease in people oroginate with the fecal discharge of infected individuals @ animals. Specific disease-producing organism presence in water are not easily identify. The techniques for comprehensive bacteriological examination are COMPLEX and TIME CONSUMING. Eg ; Total Coliform Test (Davis and Cornwell, 2008)

RADIOLOGICAL CHARACTERISTICS Caused by: The development and use of atomic energy as a power source The mining of radioactive materials Naturally occur It is necessary to establish limiting concentrations for the intake into the body. The effect of human exposure to radiation @ radioactive materials are HARMFUL and any unnecessary exposure should be avoided. The amount of radiation to which the individual is normally exposed varies with the amount of background radioactivity. Water with high radioactivity is not normal

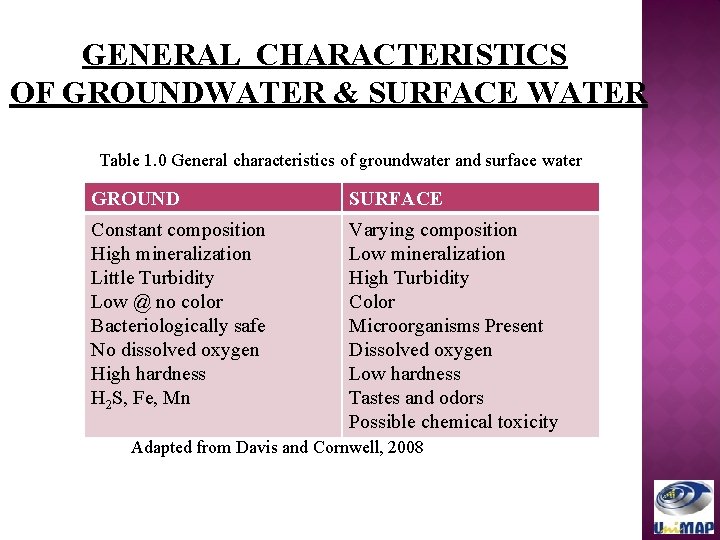
GENERAL CHARACTERISTICS OF GROUNDWATER & SURFACE WATER Table 1. 0 General characteristics of groundwater and surface water GROUND SURFACE Constant composition High mineralization Little Turbidity Low @ no color Bacteriologically safe No dissolved oxygen High hardness H 2 S, Fe, Mn Varying composition Low mineralization High Turbidity Color Microorganisms Present Dissolved oxygen Low hardness Tastes and odors Possible chemical toxicity Adapted from Davis and Cornwell, 2008

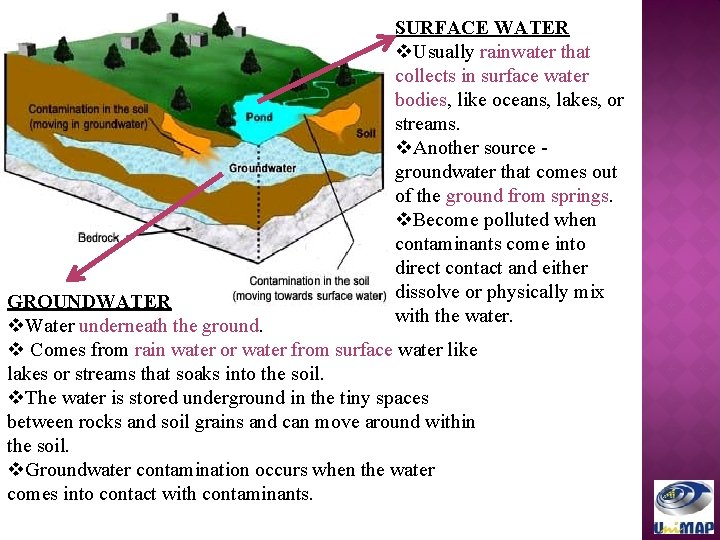
SURFACE WATER v. Usually rainwater that collects in surface water bodies, like oceans, lakes, or streams. v. Another source groundwater that comes out of the ground from springs. v. Become polluted when contaminants come into direct contact and either dissolve or physically mix with the water. GROUNDWATER v. Water underneath the ground. v Comes from rain water or water from surface water like lakes or streams that soaks into the soil. v. The water is stored underground in the tiny spaces between rocks and soil grains and can move around within the soil. v. Groundwater contamination occurs when the water comes into contact with contaminants.

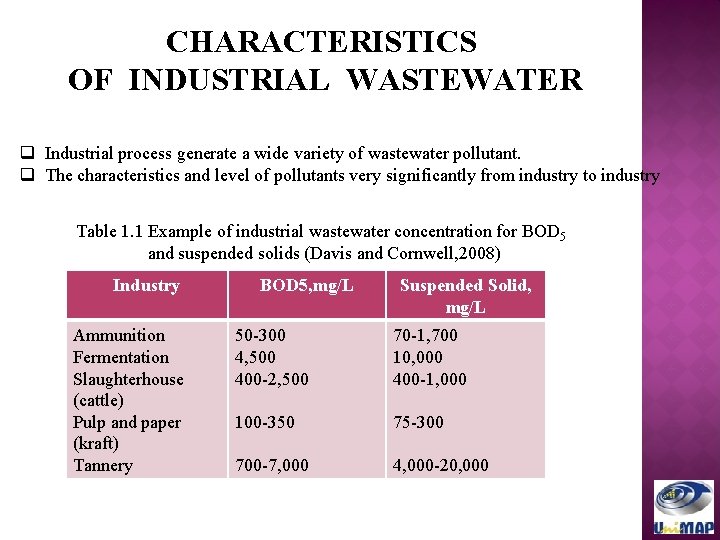
CHARACTERISTICS OF INDUSTRIAL WASTEWATER q Industrial process generate a wide variety of wastewater pollutant. q The characteristics and level of pollutants very significantly from industry to industry Table 1. 1 Example of industrial wastewater concentration for BOD 5 and suspended solids (Davis and Cornwell, 2008) Industry Ammunition Fermentation Slaughterhouse (cattle) Pulp and paper (kraft) Tannery BOD 5, mg/L Suspended Solid, mg/L 50 -300 4, 500 400 -2, 500 70 -1, 700 10, 000 400 -1, 000 100 -350 75 -300 700 -7, 000 4, 000 -20, 000

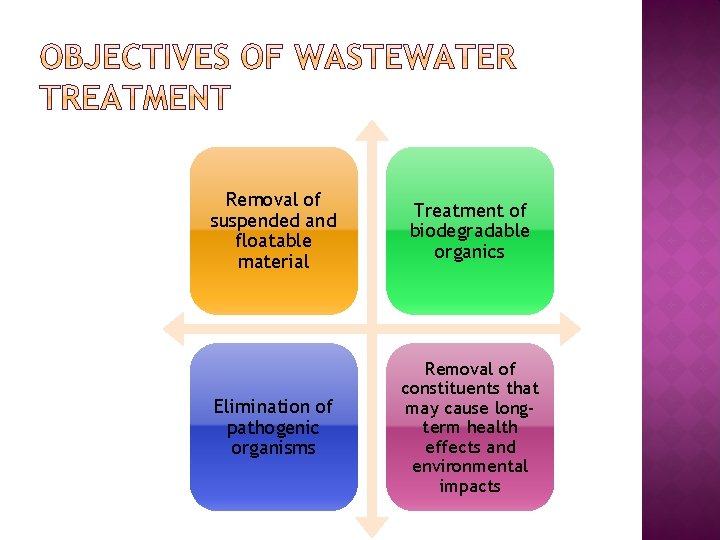
Removal of suspended and floatable material Treatment of biodegradable organics Elimination of pathogenic organisms Removal of constituents that may cause longterm health effects and environmental impacts

Chemical Physical Biological Treatments

Each treatment will further explain on next lecture… Thank You