PTT 254 MASS TRANSFER DRYING PREPARED BY PUAN

PTT 254 MASS TRANSFER DRYING PREPARED BY: PUAN KHAIRUNISSA SYAIRAH

DEFINITION OF DRYING • Removal of relatively small amount of water or organic liquids • Final processing step before packaging • As a preservative technique esp. food

METHOD OF DRYING • BATCH: - Drying process occur in a given period of time • CONTINUOUS: - Drying process occur continuously

CATEGORIES OF DRYING 1. Heat addition-Direct contact with heated air at atmospheric pressure 2. Vacuum drying - heated indirectly either by contact with a metal wall or by radiation 3. Freeze drying – sublimation process of frozen material like pharmaceuticals material

EQUIPMENTS FOR DRYING TRAY DRIER VACUUM SHELF INDIRECT DRYERS CONTINUOUS TUNNEL DRYERS ROTARY DRYERS DRUM DRYERS SPRAY DRYERS

TRAY DRYER

VACUUM SHELF INDIRECT DRYER

CONTINUOUS TUNNEL DRYER
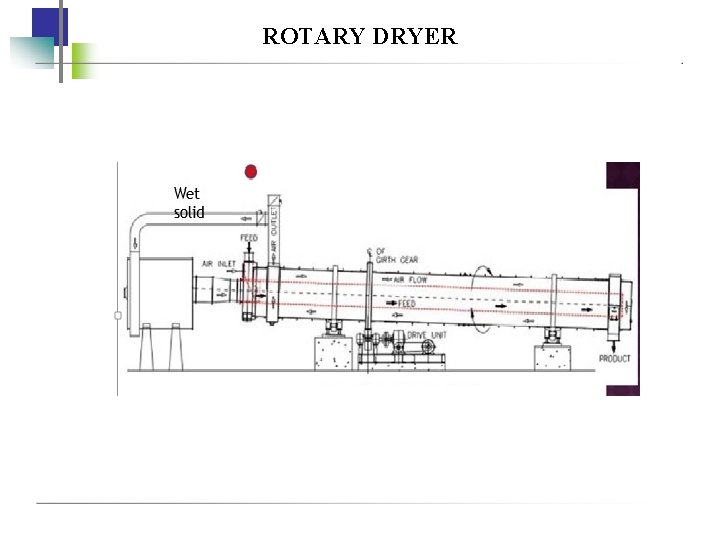
ROTARY DRYER

DRUM DRYERS

SPRAY DRYER

HUMIDIFICATION involves the transfer of water from the liquid phase into a gaseous mixture of air and water vapor. DEHUMIDIFICATION water vapor is transferred from the vapor state to the liquid state.

PHYSICAL STATES OF WATER
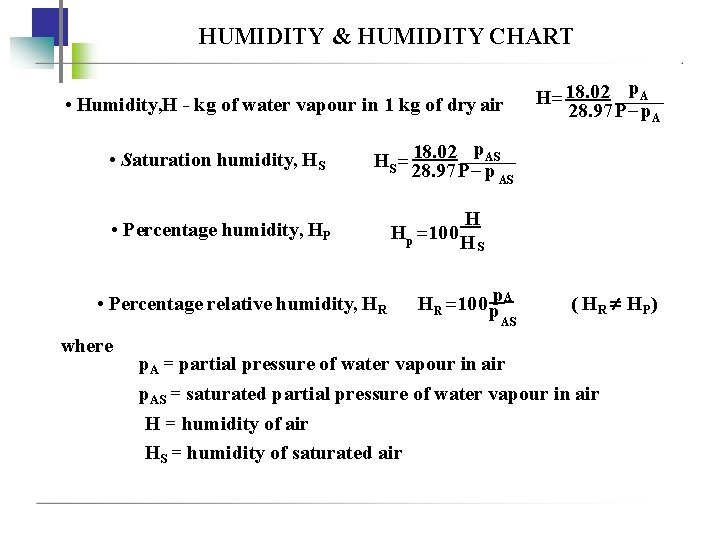
HUMIDITY & HUMIDITY CHART • Humidity, H - kg of water vapour in 1 kg of dry air • Saturation humidity, HS H 18. 02 p. A 28. 97 P p. A 18. 02 p. AS HS 28. 97 P p AS • Percentage humidity, HP • Percentage relative humidity, HR where H Hp 100 H S HR 100 pp. A AS ( H R HP ) p. A = partial pressure of water vapour in air p. AS = saturated partial pressure of water vapour in air H = humidity of air HS = humidity of saturated air
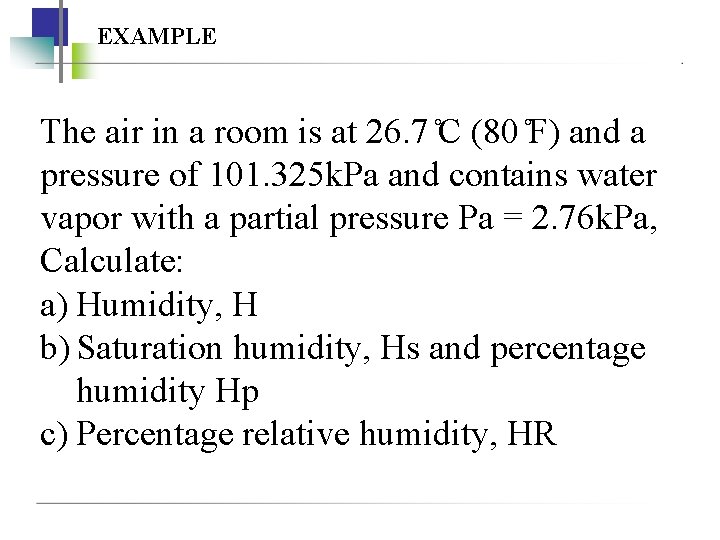
EXAMPLE The air in a room is at 26. 7 C (80 F) and a pressure of 101. 325 k. Pa and contains water vapor with a partial pressure Pa = 2. 76 k. Pa, Calculate: a) Humidity, H b) Saturation humidity, Hs and percentage humidity Hp c) Percentage relative humidity, HR
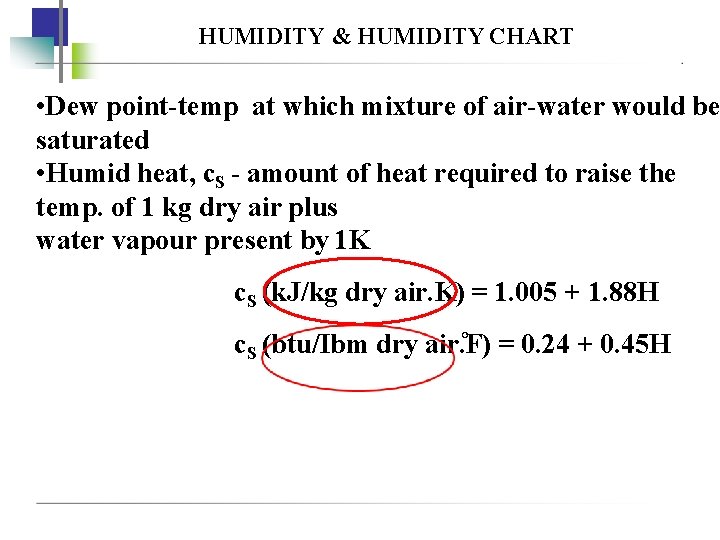
HUMIDITY & HUMIDITY CHART • Dew point-temp at which mixture of air-water would be saturated • Humid heat, c. S - amount of heat required to raise the temp. of 1 kg dry air plus water vapour present by 1 K c. S (k. J/kg dry air. K) = 1. 005 + 1. 88 H c. S (btu/Ibm dry air. F) = 0. 24 + 0. 45 H

• Humid volume, υH - total volume of 1 kg dry air plus water vapour present at 1 atm & given gas temperature υH (m 3/kg dry air) = (2. 83 x 10 -3 + 4. 56 x 10 -3 H)T K υH (ft 3/Ibm dry air) = (0. 0252 + 0. 0405 H)T R • Total entahlpy of 1 kg of air plus its water vapour, Hy Hy (k. J/kg dry air) = (1. 005 + 1. 88 H)(To. C- 0) + 2501. 4 H
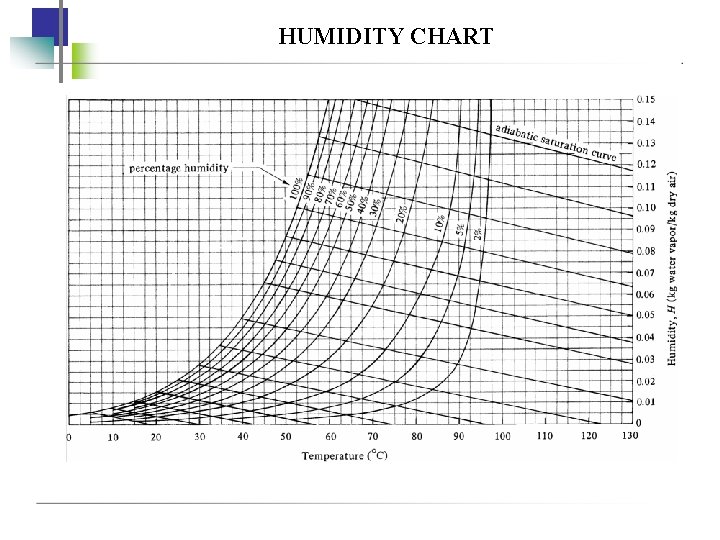
HUMIDITY CHART

EXAMPLE Air entering a dryer has a temperature (dry bulb temperature) of 60 C (140 F) and a dew point of 26. 7 C(80 F). Using the humidity chart, determine the actual humidity , H, percentage humidity Hp, humid heat cs, and humid volume υH in SI and English units.

Example on adiabatic saturation An air stream at 87. 8 C having a humidity H = 0. 030 kg H 2 O/kg dry air is contacted in an adiabatic saturator with water. It is cooled and humidified to 90 % saturation, a) What are the final values of H and T? b) For 100% saturation, what would be the values if H and T?

DRY & WET BULB TEMPERATURE wet cloth/wick Air flow Dry bulb temperature: the ordinary temperature you measure with a thermometer Wet-bulb temperature : decreases in temperature below the dry-bulb temperature until the rate of heat transfer from the warmer air to the wick is just equal to the rate of heat transfer needed to provide for the evaporation of water from the wick into the air stream.

example A water vapor-air mixture having a dry bulb temperature of T=60 C is passed over a wet bulb and the wet bulb obtained is T w = 29. 5 C, what is the humidity of the mixture?
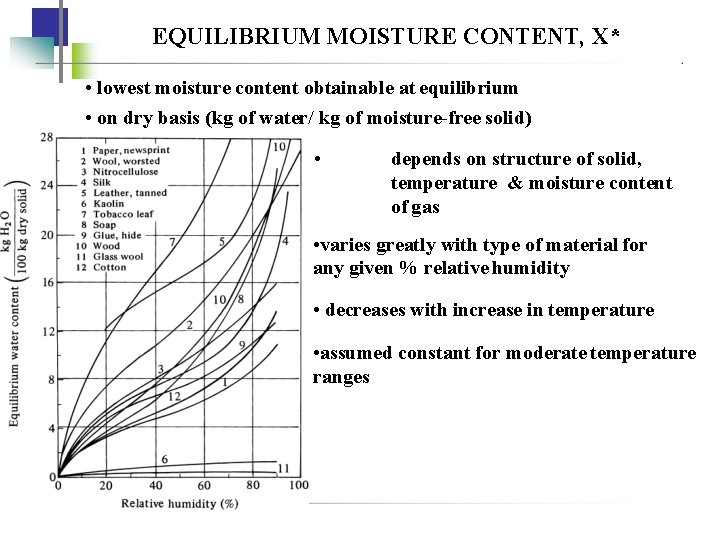
EQUILIBRIUM MOISTURE CONTENT, X* • lowest moisture content obtainable at equilibrium • on dry basis (kg of water/ kg of moisture-free solid) • depends on structure of solid, temperature & moisture content of gas • varies greatly with type of material for any given % relative humidity • decreases with increase in temperature • assumed constant for moderate temperature ranges

• bound water - the minimum moisture a material can carry - intersection of 100% humidity line in equillibrium water content vs relative humidity -the material will known as hygroscopic material • Unbound water = excess water held primarily in the voids of the solid • free moisture content, X - moisture above the equilibrium moisture content - can be removed by dryin X* = equilibrium-moisture content

RATE OF DRYING CURVES • batch drying • experimental determination data : WS = weight of dry solid W total weight of wet solid vs time t To obtain as free moisture content X vs time t: W WS total moisture content , Xt = WS free moisture content, X = Xt - X* To obtain as rate of drying, R : Get slopes of tangents at different values of t : R Ws d. X A dt where A = exposed surface area for drying

RATE OF DRYING CURVES Point AB : Warming up (unsteady) period where the solid surface conditions come into equilibrium with the drying air. Point A’ : hot solid Point B-C: constant-rate drying period in which surface of the solid remains saturated with liquid because the movement of water vapour to the surface equals the evaporation rate. Thus the drying rate depends on the rate of heat transfer to the drying surface and temperature remains constant. Surface temperature TW Point C : critical free moisture content, XC , where the drying rate starts falling and surface temperature rises. Insufficient water on surface

RATE OF DRYING CURVES Point C-D : first falling-rate drying period which surface is drying out. Rate of water to surface is less that rate of evaporation from surface Point D : surface completely dry Point D-E : second falling-rate period in which evaporation is from inside of solid. Point E : equilibrium moisture content, X*, where no further drying occur

CONSTANT RATE OF DRYING PERIOD To determine the time required for drying from X 1 to X 2: 1. Experimental drying curve 2. Predicted mass-and-heat coefficients Experimental drying curve: Under similar conditions to actual process 1. Drying curve X vs t 2. Rate-of-drying curve R vs X where t WS (X 1 X 2) ARC RC = constant rate of drying WS = kg of dry solid used A = exposed surface area for drying

example A solid whose drying curve is represented by drying curve graph is to be dried from a fee moisture content X 1= 0. 38 kg H 20/kg dry solid to X 2=0. 25 kg H 20/kg dry solid. Estimate the time required.
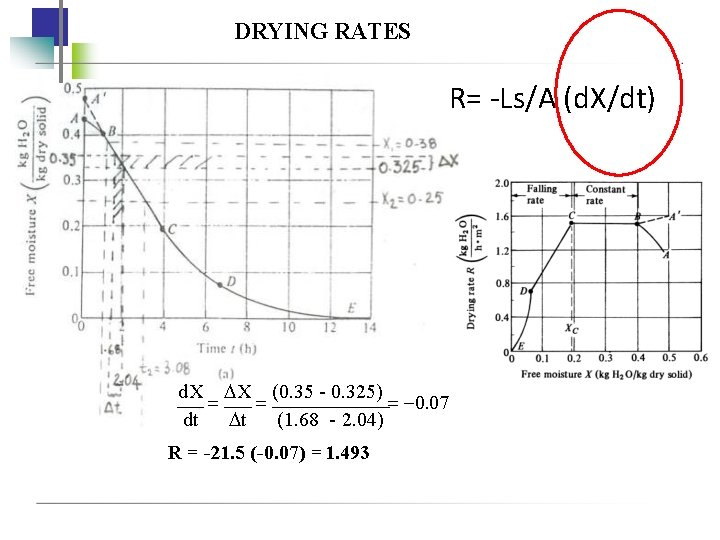
DRYING RATES R= -Ls/A (d. X/dt) d. X X (0. 35 - 0. 325) 0. 07 dt t (1. 68 - 2. 04) R = -21. 5 (-0. 07) = 1. 493

FALLING-RATE OF DRYING PERIOD To determine the time required for drying from X 1 to X 2: 1. Numerical integration W t A S X 1 X d. X W X 1 R R A S 2 Most accurate av
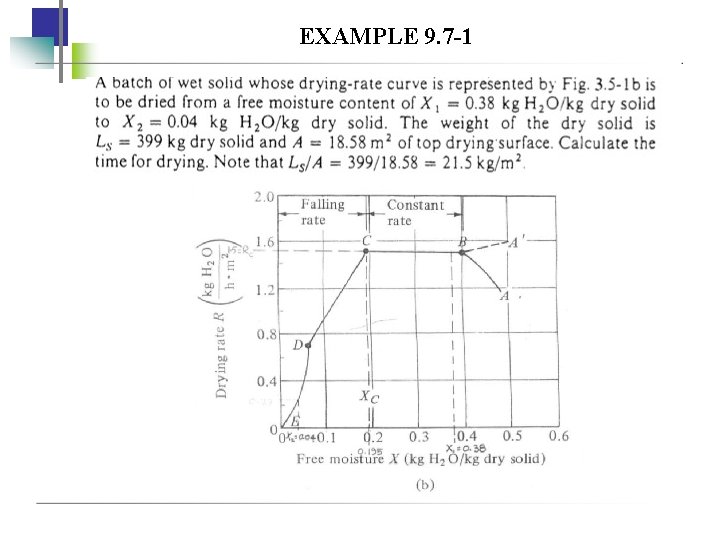
EXAMPLE 9. 7 -1
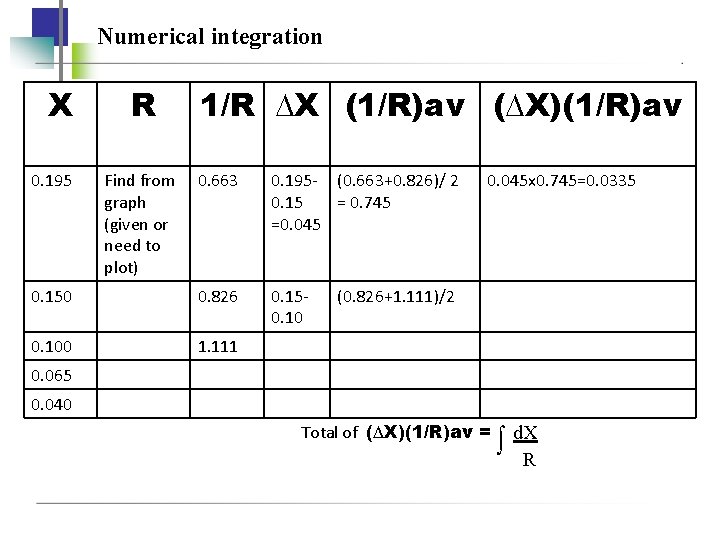
Numerical integration X 0. 195 R Find from graph (given or need to plot) 1/R ∆X (1/R)av (∆X)(1/R)av 0. 663 0. 195 - (0. 663+0. 826)/ 2 0. 15 = 0. 745 =0. 045 0. 150 0. 826 0. 150. 100 1. 111 0. 045 x 0. 745=0. 0335 (0. 826+1. 111)/2 0. 065 0. 040 Total of (∆X)(1/R)av = d. XR

FALLING-RATE OF DRYING PERIOD To determine the time required for drying from X 1 to X 2: 2. Special cases a) Rate is linear function of X t WS(X 1 X 2) R 1 ln A(R 1 R 2) R 2

FALLING-RATE OF DRYING PERIOD To determine the time required for drying from X 1 to X 2: 2. Special cases b) Rate is a linear function thru’ origin (a straight line from C to E at the origin) R t WSXC ln C ARC R 2 or X t WSXC ln C ARC X 2 and R RC X XC
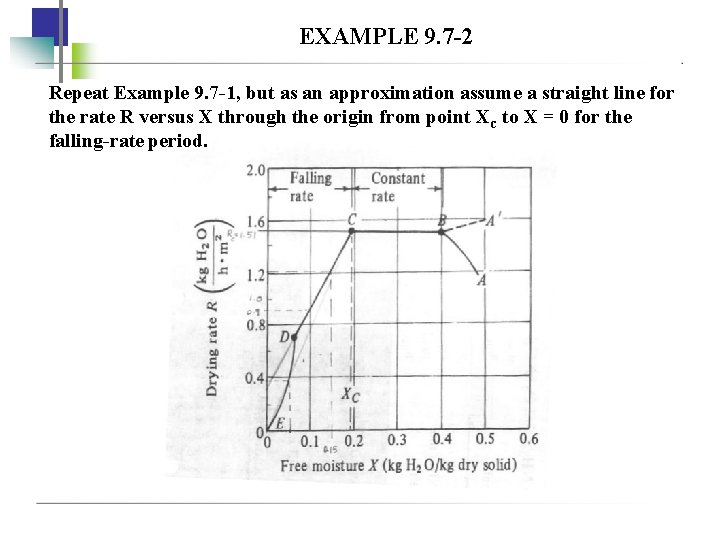
EXAMPLE 9. 7 -2 Repeat Example 9. 7 -1, but as an approximation assume a straight line for the rate R versus X through the origin from point Xc to X = 0 for the falling-rate period.

MECHANISM OF DRYING Liquid diffusion • occurs when there is a concentration difference between the depths of the solid and the surface. • found in nonporous solid like glue. • Moisture diffusivity decrease with decreasing moisture content. Capillary movement in porous solids • involves surface tension. • meniscus of liquid water forms across each pore in the depth of the solid and creates capillary force by the interfacial tension between the water and the solid (driving force). • smaller pores develop greater force than large pores
- Slides: 37