PTT 205 HEAT MASS TRANSFER SEM II 20162017

















- Slides: 22

PTT 205 HEAT & MASS TRANSFER SEM II (2016/2017) PRINCIPLES OF MASS TRANSFER (PART 2)

MOLECULAR DIFFUSION IN LIQUIDS Lecture Content: Introduction Case 1: Equimolar counter diffusion Case 2: Diffusion of A through non-diffusing B Diffusion coefficients for liquids Prediction of diffusivities in liquids

Introduction u Typical phenomena of molecular diffusion in liquids: -liquid extraction -solvent extraction -diffusion of salt in blood u Molecular diffusion in liquid is smaller than molecular diffusion in gases because molecules in liquid are closed together compared to molecules in gas. u Thus, molecules of diffusing solute will collide more frequently with liquid molecules. u Diffusivity in liquid depens on the concentration of diffusing solute

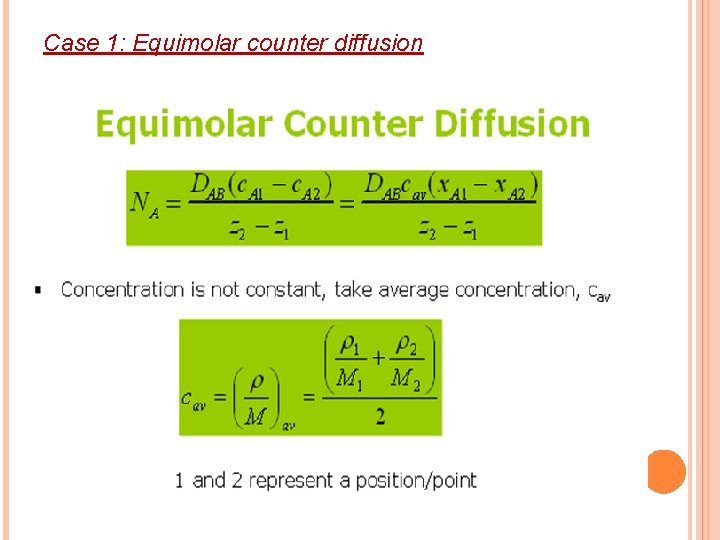
Case 1: Equimolar counter diffusion

Case 2: Diffusion of A through nondiffusing B * For dilute solution; XBM is close to 1 and c is constant, thus;

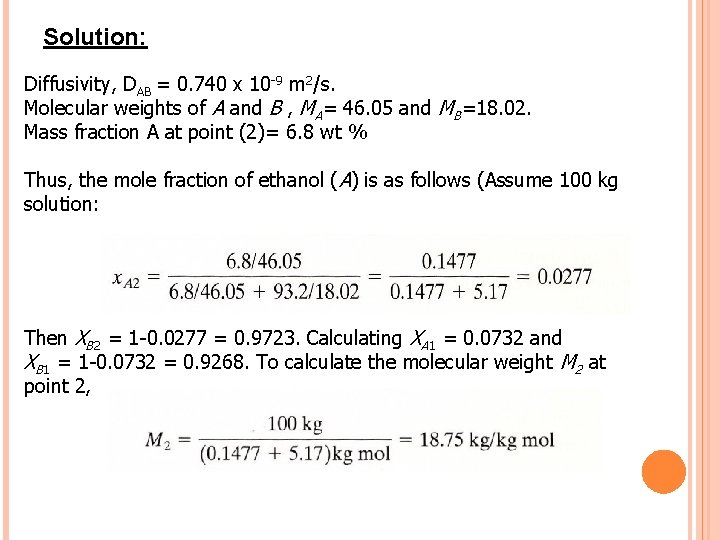
Case 1: Diffusion of A through nondiffusing B Example 1: Diffusion of Ethanol (A) through Water (B) An ethanol (A)-water (B) solution in the form of a stagnant film 2. 0 mm thick at 293 K is in constant at one surface with an organic solvent in which ethanol is soluble and water is insoluble. Hence, NB = 0. At point 1 the concentration of ethanol is 16. 8 wt % and the solution density is ρ1 = 972. 8 kg/m 3. At point 2 the concentration of ethanol is 6. 8 wt % and ρ2 = 988. 1 kg/m 3. The diffusivity of ethanol is 0. 740 x 109 m 2/s. Calculate the steady-state flux, NA.

Solution: Diffusivity, DAB = 0. 740 x 10 -9 m 2/s. Molecular weights of A and B , MA= 46. 05 and MB=18. 02. Mass fraction A at point (2)= 6. 8 wt % Thus, the mole fraction of ethanol (A) is as follows (Assume 100 kg solution: Then XB 2 = 1 -0. 0277 = 0. 9723. Calculating XA 1 = 0. 0732 and XB 1 = 1 -0. 0732 = 0. 9268. To calculate the molecular weight M 2 at point 2,

Solution: Similarly, M 1 = 20. 07. To calculate XBM we can use the linear mean since XB 1 and XB 2 are close to each other: Substituting into equation and solving,

Diffusion coefficients for liquids

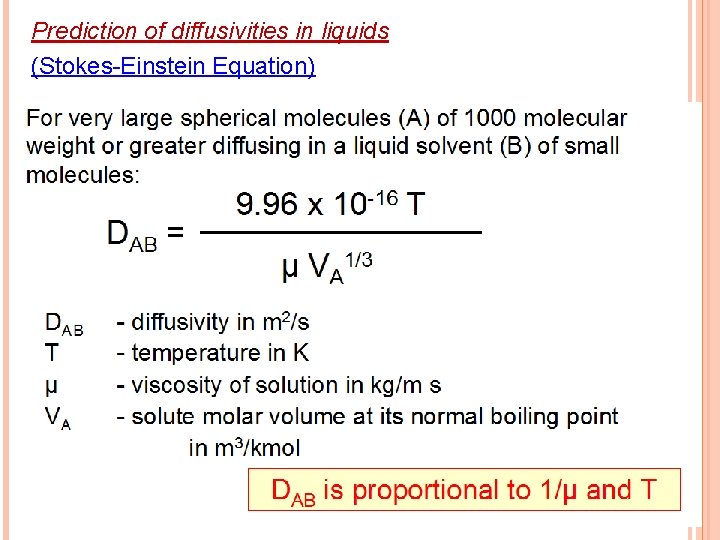
Prediction of diffusivities in liquids (Stokes-Einstein Equation)

Prediction of diffusivities in liquids (Wilke-Chang Equation) DAB - diffusivity in m 2/s MB - molecular weight of solvent B T - temperature in K μ - viscosity of solvent B in kg/m s VA - solute molar volume at its normal boiling point in m 3/kmol Φ - association parameter of the solvent, which 2. 6 for water, 1. 9 for methanol, 1. 5 for ethanol, and so on

MOLECULAR DIFFUSION IN BIOLOGICAL MATERIALS Content: Diffusion of biological solutes in liquids � Diffusion Coefficient for Biological Solutes in Aqueous Solution � Prediction of Diffusivity for Biological Solutes Diffusion of solutes in biological gels

Diffusion of biological solutes in liquids Example of applications: Food Processing Diffusion of volatile constituents in food materials through the liquid during evaporation. Fermentation Process Diffusion of nutrients, sugar, oxygen to microorganisms. Diffusion of waste product and enzyme microorganisms. the from Medical Application Dialysis machine - Diffusion of various waste products from blood to a membrane and through membrane to an aqueous solution.

Diffusion Coefficient for Biological Solutes in Aqueous Solution

Prediction of Diffusivity for Biological Solutes Wilke-Chang Equation § § Small solutes in aqueous solution with molecular weight less than 1000 or solute molar less than about 0. 500 m 3/kmol Refer to liquid diffusion part Stokes-Einstein Equation § § For larger solutes Refer to liquid diffusion part Modified Polson Equation § § § For a molecular weight above 1000 MA= MW for large molecule A Suitable for sphere-shaped molecules

Prediction of Diffusivity for Biological Solutes Example 2 : Prediction of diffusivity of albumin Predict the diffusivity of bovine serum albumin at 298 K in water as a dilute solution using the modified Polson equation and compare with the experimental value in Table 6. 4 -1.

Solution: The molecular weight of bovine serum albumin (A) From Table 6. 4 -1 is MA= 67500 kg/kg mol. The viscosity of water at 25 °C is 0. 8937 x 10 -3 Pa. s at T= 298 K. Substituting into Polson Equation This value is 11% higher than the experimental value of 6. 81 x 10 -11 m 2/s

Diffusion in Biological Gels q Gels: semisolid materials which are ‘porous’. q Examples: agarose, agar, gelatin. q Composition: macromolecules in dilute solution with the gel comprising a few wt% of the water sol. q “Pores”: usually are filled with water. q Rate of diffusion of small solutes in the gels are less than in aqueous solution. q Why? : Gel structure is to increase the path length of diffusion.

Diffusivities of solutes in dilute biological gels in aqueous sol.

Diffusivity of solutes in biological gel Example 3 : Diffusion of urea in agar A tube or bridge of a gel solution of 1. 05 wt% agar in water at 278 K is 0. 04 m long and connects two agitated solutions of urea in water. The urea concentration in the first solution is 0. 2 g mol urea per liter solution and is 0 in the other. Calculate the flux of urea in kg mol/s. m 2 at steady state.

Solution: From Table 6. 4 -2, the DAB of urea at 278 K is 0. 727 X 10 -9 m 2/s. For urea diffusing through stagnant water in the gel; use the bottom Equation; Since the value of x. A 1 is less than about 0. 01, the solution is quite dilute and x. BM = 1. Hence, we can use the equation; Given that CA 1 = 0. 2/1000 = 0. 0002 g mol /cm 3 = 0. 2 kg mol/m 3 and CA 2 = 0, substituting into the above equation;

THANK YOU