PTRT 1309 CORROSION BASICS Chapter 1 PETEX Book

PTRT 1309 CORROSION BASICS Chapter 1 – PETEX Book Oil and Gas Technology Program
Objectives • Define reduction and oxidation • Explain the difference between base metals and noble metals • Define electrolyte • Name the components of a corrosion cell • Describe the various chemical reactions associated with common corrosion cells • Define anode and cathode • Explain why corrosion cell action is said to be cathodically controlled • Name the various types of corrosion cells and explain how they occur. Oil and Gas Technology Program

THE CORROSION PROCESS • • • CORROSION IS THE DETERIORATION OF MATERIAL BY A CHEMICAL OR ELECTRO-CHEMICAL REACTION WITH ITS ENVIRONMENT The most common metal in oil production operations is steel ENVIRONMENTAL FACTORS ARE OF TWO TYPES. OUTSIDE AND INSIDE – OUTSIDE PIPE AND FACILITY ENVIRONMENTS: • AIR • WATER • AND SOILS – INSIDE ENVIRONMENTS • PRODUCED FLUIDS – OIL – GAS – WATER • CHEMICALS • GLYCOLS Oil and Gas Technology Program

External Environments • Atmospheric Corrosion (most to least severe) – – – Industrial and marine or seacoast Industrial and high humidity Marine or seacoast High humidity only Inland, industrial low humidity Rural, non-industrial, low humidity • Soil Corrosion – factors affecting severity – – Moisture – how wet is the soil, swamp, or dry sand dunes Salts – naturally or brine Temperature of the soil at the pipe surface Oxygen availability Oil and Gas Technology Program
Corrosion Process • THE BASIC CORROSION CELL HAS FOUR PARTS – ANODE – REPRESENTED AS A NEGATIVE TERMINAL OF THE CELL – CATHODE – REPRESENTED AS THE POSITIVE TERMINAL OF THE CELL – ELECTROLYTE – A CHEMICAL, THAT WHEN DISSOLVES IN WATER, DISSOCIATES INTO POSITIVE AND NEGATIVE IONS, THUS INCREASING THE ELECTRICAL CONDUCTIVITY – METAL • • • CORROSION IS A CIRCUIT. IF THE CIRCUIT IS INTERUPTED IN ANY WAY CORROSION CAN BE CONTROLLED DRIVING FORCE – (Created by a voltage difference between metals) A COMPLETE ELECTRICAL CIRCUIT – Electrolyte – conductor Oil and Gas Technology Program
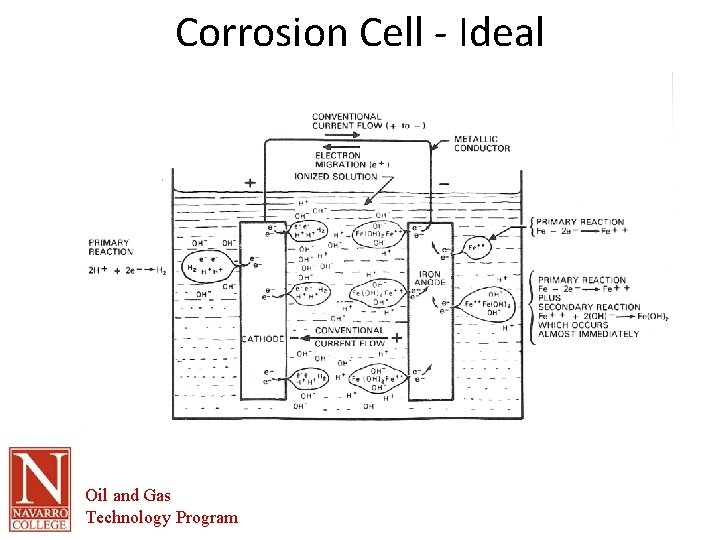
Corrosion Cell - Ideal Oil and Gas Technology Program
Basic Electrical Theory Electrical current flow is defined as the movement of a charged particle (Electrons) in a specified direction. These charged particles can move through varying materials – • • Metallic Conductors- electrons flow through a conductor (metals). There is no detrimental effect on the conductor as the electrons are not lost Electrons go from one atom of the metal to the other. Electrolytic- Electron move through the liquid via Ions (charged particles). In our corrosion cell metal is lost where current is discharged into an electrolyte because the loss of an electron breaks the bonds that hold the metal together. Oil and Gas Technology Program
Basic Electrical Theory Units of Measurement Voltage- A unit of measurement which measures the difference in potential. Electrical pressure or differential pressure (PSI). – • Voltage can be measured without breaking the circuit (parallel). Oil and Gas Technology Program
Basic Electrical Theory Units of Measurement Ampere (amps) - A unit of measurement of electrical flow rate…. . How many electrons go through the circuit over a known time period? Just like speed is measured in miles per hours. Correlation- MMCFD, gallons/ minute, etc. – • • • The Circuit must be broken in order to measure amperage (series measurement) Amperage is usually measured indirectly through application of ohms law. Similar to natural gas flow measurement. Oil and Gas Technology Program
Basic Electrical Theory Units of Measurement Ohms- a unit used to quantify electrical resistance to flow – • • In electricity it’s the size of conductor or size of load Oil and gas correlation – – pipe size chokes Oil and Gas Technology Program
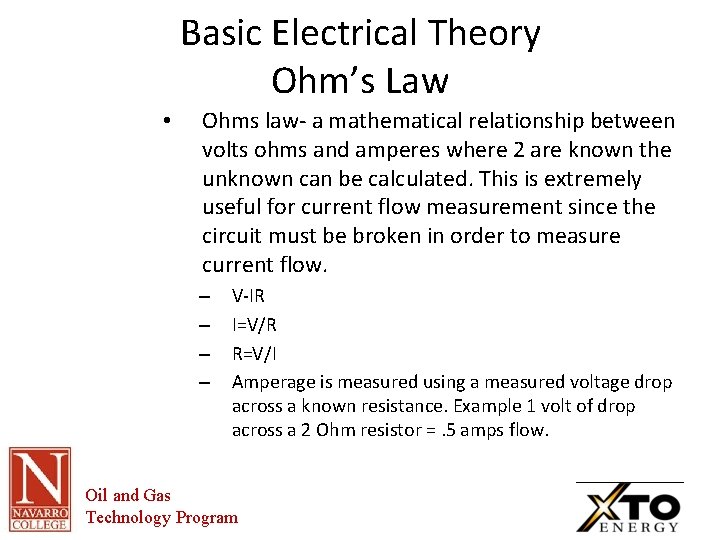
Basic Electrical Theory Ohm’s Law • Ohms law- a mathematical relationship between volts ohms and amperes where 2 are known the unknown can be calculated. This is extremely useful for current flow measurement since the circuit must be broken in order to measure current flow. – – V-IR I=V/R R=V/I Amperage is measured using a measured voltage drop across a known resistance. Example 1 volt of drop across a 2 Ohm resistor =. 5 amps flow. Oil and Gas Technology Program
Basic Electrical Theory – – Polarity- defines a charge as positive or negative. Like charges repel opposing charges attract Direction of flow- The direction the current flow in an electrical circuit Oil and Gas Technology Program
Basic Electrical Theory Alternating Current – – – Alternating Current (AC) AC is the type of electrical power that is commercially available When discussing AC circuits flow direction is not an issue as the electrons go back an fourth along the conductor The primary reason AC is used so widely is the ease of electrical transmission The electrons do not have to “move” long distances therefore reducing loss. Oil and Gas Technology Program
Basic Electrical Theory Direct Current (DC) – – – Direct Current (DC) - Current flows in one direction the electrons have to move through the circuit from start to finish This causes power loss over long distances rendering it inefficient for long distance transmission When we discuss the basic corrosion cell it is DC. Oil and Gas Technology Program

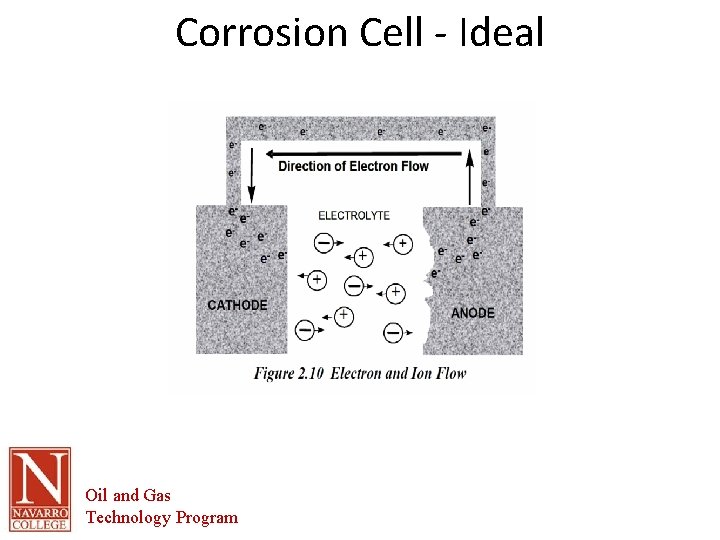
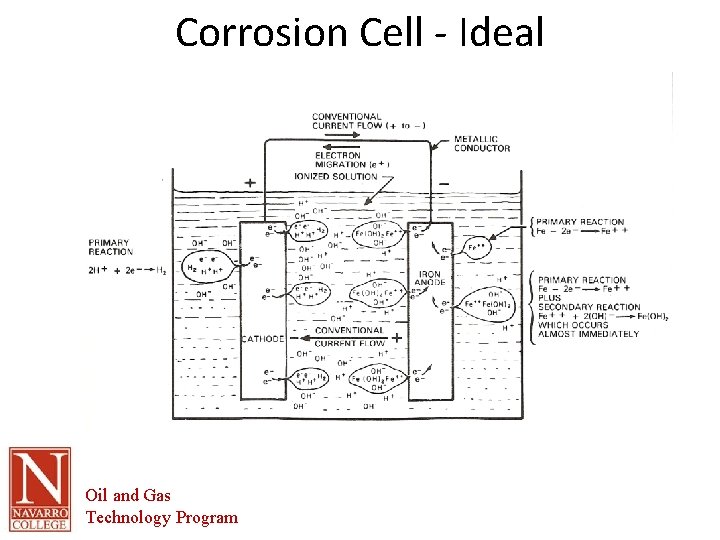
The Corrosion Cell • Corrosion cell- the corrosion cell has 4 parts. – – • • • Anode Cathode Electrolyte Metallic Path All 4 parts must be present in order for the material to corrode. Removal of one part of the cell renders it inactive All corrosion mitigation methods are based on this premise. Oil and Gas Technology Program
The Corrosion Cell • Anode – The area of the corrosion cell where metal loss occurs – Where oxidation occurs. – Oxidation is the loss of electrons – The loss of electrons causes the material to break down or corrode. Oil and Gas Technology Program
The Corrosion Cell • Cathode – The area of the corrosion cell where NO metal loss occurs – Where Reduction occurs. – Reduction is the gain of electrons – This gain in electrons creates protective mineral deposits due to the chemical reaction occurring at the cathodic surface Oil and Gas Technology Program
The Corrosion Cell Electrolyte- An electrically conductive solution • • • – – – – • For a solution to e conductive it must contain ions. The more Ions the more conductive or corrosive the solution. Ions- charged particles that can transport electrical current through the solution Examples in Oil and Gas production Produced water Sea water Fresh water Soil Electrolyte- in most scenarios this is the part of the corrosion cell that is utilized for control of corrosion. This is done through barrier coatings of chemically altering the electrolyte. Water is the primary electrolyte. All electrolytes are electrically conductive. Pure water is not conductive, but the addition of minerals makes it conductive. Oils are not conductive by their nature. Oil and Gas Technology Program
The Corrosion Cell • – Some factors that effect the corosivity of an electrolyte • • • – • • • Water- pure water is a POOR conductor. Minerals must be present to support current flow Soils Salt Content- produced fluids Ionic concentration p. H Dissolved Oxygen Temperature Salt content Moisture content Soil make up relating to electrical resistivity – • • • – • • • Sand has a high electrical resistance while clays are low. p. H Oxygen concentration Temperature Atmospheric Corrosion (most to least severe) Industrial and marine or seacoast Industrial and high humidity Marine or seacoast High humidity only Inland, industrial low humidity Rural, non-industrial, low humidity Oil and Gas Technology Program
The Corrosion Cell Metallic path- a metallic connection between the anode and cathode In the oil and gas industry this part of the cell is inherent in the structure to be protected. • • – – Anodic and cathodic areas are part of the pipe Therefore the anodes and cathodes cannot be electrically separated. Oil and Gas Technology Program
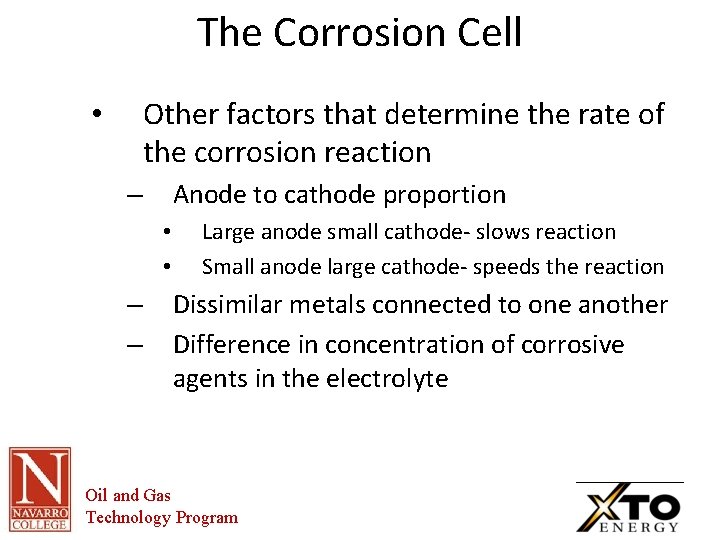
The Corrosion Cell Other factors that determine the rate of the corrosion reaction • Anode to cathode proportion – • • – – Large anode small cathode- slows reaction Small anode large cathode- speeds the reaction Dissimilar metals connected to one another Difference in concentration of corrosive agents in the electrolyte Oil and Gas Technology Program
Corrosion Cell - Ideal Oil and Gas Technology Program
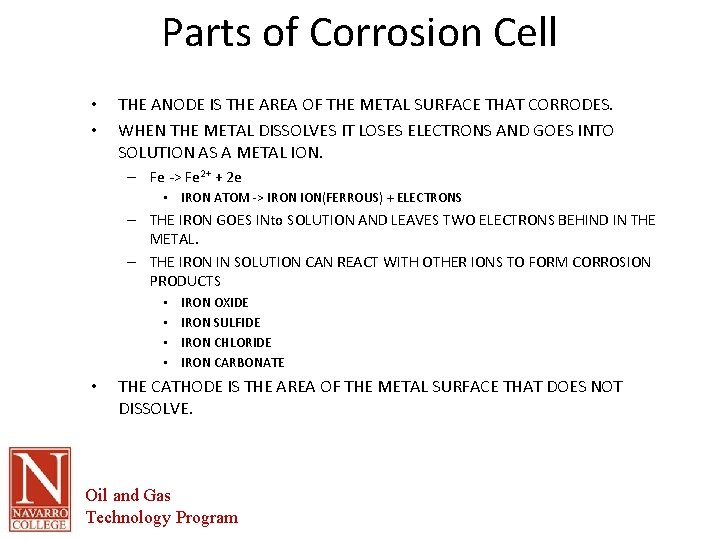
Parts of Corrosion Cell • • THE ANODE IS THE AREA OF THE METAL SURFACE THAT CORRODES. WHEN THE METAL DISSOLVES IT LOSES ELECTRONS AND GOES INTO SOLUTION AS A METAL ION. – Fe -> Fe 2+ + 2 e • IRON ATOM -> IRON ION(FERROUS) + ELECTRONS – THE IRON GOES INto SOLUTION AND LEAVES TWO ELECTRONS BEHIND IN THE METAL. – THE IRON IN SOLUTION CAN REACT WITH OTHER IONS TO FORM CORROSION PRODUCTS • • • IRON OXIDE IRON SULFIDE IRON CHLORIDE IRON CARBONATE THE CATHODE IS THE AREA OF THE METAL SURFACE THAT DOES NOT DISSOLVE. Oil and Gas Technology Program
Corrosion Cell - Ideal Oil and Gas Technology Program
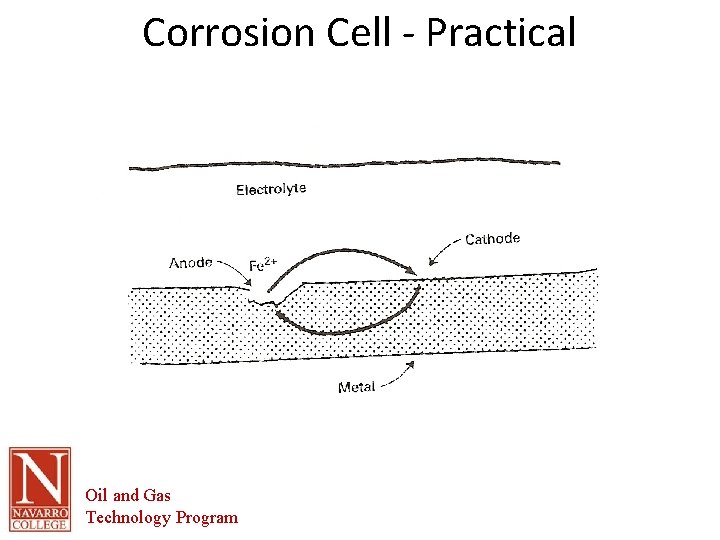
Corrosion Cell - Practical Oil and Gas Technology Program

Origins of Corrosion • • • AS IRON ORE IS PROCESSED INTO STEEL ITS ENERGY LEVEL IS INCREASED (Reduction) IRON AND STEEL CORRODE BECAUSE THE METAL IS ALWAYS TRYING TO RETURN TO ITS LOWEST LEVEL OF ENERGY (Thermodynamics) THE SOURCE OF VOLTAGE IN THE CORROSION PROCESS COMES FROM THE different amount of ENERGY STORED IN THE METAL DURING REFINING DIFFERENT METALS REQUIRE VARYING AMOUNTS OF ENERGY FOR REFINING THEREFORE HAVE DIFFERENT TENDENCIES TO CORRODE THE VOLTAGE OF A METAL CAN BE DETERMINED BY THE DIFFERENCE IN POTENTIAL ENERGY BETWEEN THE STANDARD HYDROGEN ELECTRODE AND THE METALS POTENTIAL. THE MOST REACTIVE METALS ARE BASE METALS – POTASSIUM – CALCIUM – SODIUM Oil and Gas Technology Program

Origins of Corrosion • THE LEAST REACTIVE METALS ARE NOBLE METALS – GOLD – PLATINUM – MERCURY • • THE POTENTIAL OF THE METAL IS THE SIZE OF THE DRIVING VOLTAGE GENERATED BY THE METAL IN an aqueous SOLUTION THE POTENTIAL OF THE METAL IS RELATED TO THE ENERGY RELEASED WHEN THE METAL CORRODES THE ABSOLUTE VALUE OF THE POTENTIAL IS INFLUENCED BY THE WATER COMPOSITION, TEMPRATURE, VELOCITY, AND MANY OTHER FACTORS. HOWEVER, THEIR RELATIVE VALUES REMAIN ABOUT THE SAME IN MOST PRODUCTION WATERS BESIDES this SOURCE OF VOLTAGE THERE MUST BE A COMPLETE ELECTRICAL CIRCUIT CONSISTING OF AN ANODE, CATHODE, AND AN ELECTROLYTE Oil and Gas Technology Program

CORROSION CELL SIZE • • LARGE CELLS LIKE THOSE ALREADY DISCRIBED CAN CAUSE LARGE QUANTITIES OF METAL LOSS. LARGE CELLS USUALLY INVOLVE MASSIVE AMOUNTS OF ELECTROLYTES – SOIL MOISTURE – LARGE BODIES OF WATER • SMALL CELLS CAN BE SMALL ENOUGH TO BE BETWEEN ADJACENT GRAINS OF METAL WITH DIFFERENT POTENTIALS – SMALL CELLS CAN BE VARY EFFICIENT WITH A LOW RESISTANCE AND RELATIVELY LARGE CURRENT AVAILABLE • Beware of small anodes or large cathodic areas – Rapid electron transfer – High corrosion rates Oil and Gas Technology Program
Justification for Corrosion Mitigation Asset life expectancy- design life – • • • will the asset last long enough to justify the cost of remediation Cost-Results of the study show that the total annual estimated direct cost of corrosion in the U. S. is a staggering $276 billion— approximately 3. 1% of the nation’s Gross Domestic Product (GDP). Safety and environmental concerns – – – Affected public safety Employee safety Environmental contamination Oil and Gas Technology Program

Justification for Corrosion Mitigation – • Regulatory consideration- This is an industry that is heavily regulated by both state and federal agencies. PHMSA – • EPA – • Regulates corrosion control methods and standards for pipelines based their proximity to population and/ or the materials transported Regulates environmental standards. Leaks caused by corrosion get their attention State agencies (Texas Railroad Commission, Oklahoma Corporation Commission etc. ) » » Oil and Gas Technology Program Regulate oil and gas production Regulate intrastate pipelines under the direction of PHMSA

Oil and Gas Equipment at Risk – • • – – • • • Well bore tubing casing production equipment Pipelines- Each category within the pipeline industry has unique inherent risks to integrity Gathering- Internal corrosion Transmission- External Corrosion Distribution- Third party damage/ External corrosion Process Equipment Vessels Processing plant piping Storage tanks Oil and Gas Technology Program

Oil and Gas Equipment at Risk Corrosion prevention is of special importance to the oil and gas industry – • • • Flammable materials Pressurized equipment- the unintended release of pressure form this equipment is usually violent and dangerous Environmentally sensitive materials High cost of materials and equipment Value of product lost. Safety of employees and effected public Oil and Gas Technology Program
External Corrosion that occurs from the soil or atmospheric side of the equipment Mitigation Methods – – • Cathodic protection – – – • requires the surface to be immersed in a continuous electrolyte Does not stop corrosion dictates the anodic area Protects the pipe at coating holidays Protective coatings – – – THERE IS NO PERFECT COATING Form a protective barrier between the surface and the electrolyte Some coatings (IE galvanizing) utilize anodic material in the coating to create a protective current. Oil and Gas Technology Program

FBE Coating Failure Oil and Gas Technology Program
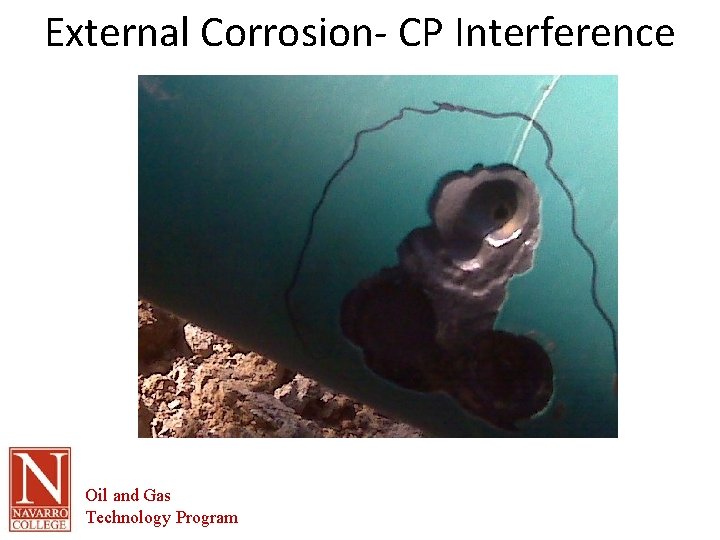
External Corrosion- CP Interference Oil and Gas Technology Program
Internal Corrosion – • Internal Corrosion- corrosion that occurs inside of the equipment as a result of the corrrosivity of the material held in the equipment. Localized (pitting) - corrosion that is concentrated in a small area. These mechanisms typically go through the wall of the vessel, pipe, or tank quickly. – – • Acid gasses » H 2 S » CO 2 MIC- Microbial induced corrosion » SRB » APB General- metal is lost over the entire surface of the material. This can be combated based on the life of the asset and thickness of the material. Oil and Gas Technology Program

Oil and Gas Technology Program

CO 2 Corrosion Oil and Gas Technology Program

H 2 S Corrosion Oil and Gas Technology Program

SRB- Tank Bottom Oil and Gas Technology Program
Internal Corrosion Mitigation • – Mitigation Methods • • • – Corrosion Inhibiting chemicals Water soluble- dissolve in the corrosive media reducing the corrrosivity of the water (electrolyte Oil soluble- coat the inside of the pipe forming a barrier Biocide- kills corrosive bacteria Design- This can cover a broad range of topics Material selection- Use a material that is not attacked by its environment Eliminate stagnant area Use thicker materials- only effect when dealing with general forms of corrosion Design for easy replacement- Adding bypasses, isolation valves etc. Pigging Used to displace free liquids from the pipeline Chemical application Internal coatings- form a barrier between surface and electrolyte Typically ineffective Oil and Gas Technology Program

10” Brush Pig Oil and Gas Technology Program

10” Poly Pig Oil and Gas Technology Program

General Causes of Corrosion • • • Oxygen concentration Galvanic action Stress Stray currents Erosion Nonuniform surfaces Oil and Gas Technology Program

Oxygen-Concentration • Perhaps the most common concentration cell. • Occurs when the electrolyte contains varying amounts of dissolved oxygen. • When oxygen has access to a moist metal surface, corrosion is promoted. However, it is promoted the most where the oxygen concentration is the least. • Electron flow in same direction of other cells and may interact with and intensify action of other cells. • As a result, sections of a metal that are covered by dirt or scale will often corrode faster, since the flow of oxygen to these sections is restricted. An increased corrosion rate will lead to increased residue, further restricting the oxygen flow to worsen the situation. Pitting often results from this "runaway" reaction. Oil and Gas Technology Program
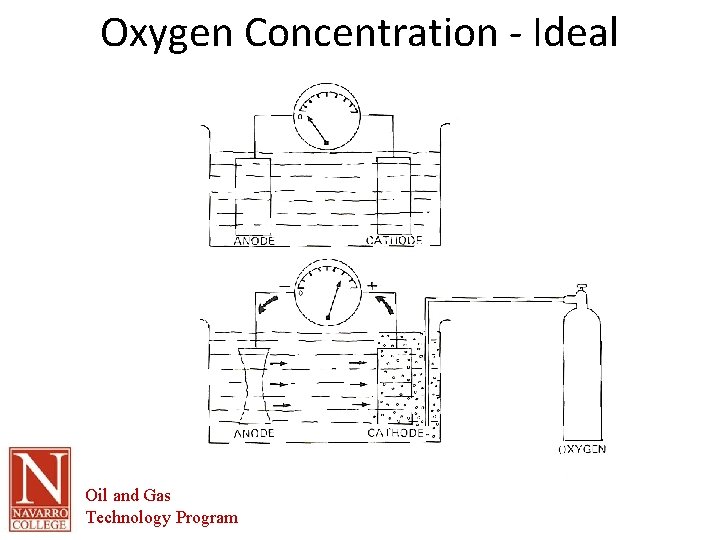
Oxygen Concentration - Ideal Oil and Gas Technology Program
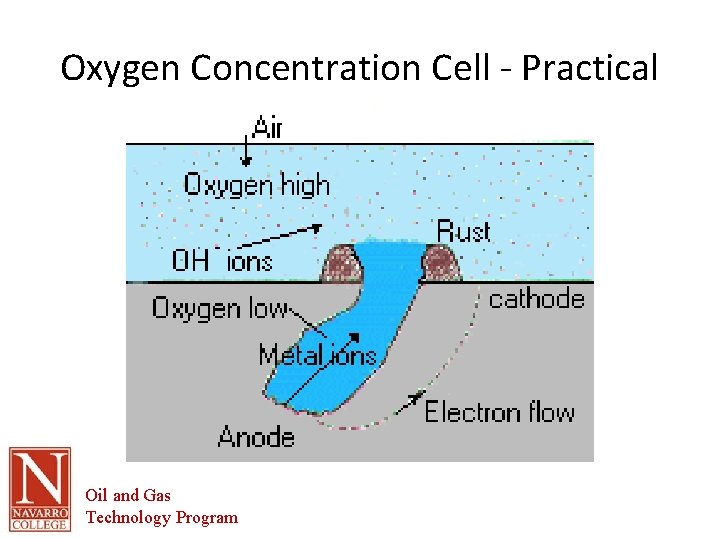
Oxygen Concentration Cell - Practical Oil and Gas Technology Program

Galvanic Cells • First utilized by Luigi Galvani to make batteries. • Caused by the difference in electrical potentials of dissimilar metals. • The potential is equal to the difference in the potential values for the two metals. • Anode will be higher in electromotive series. Oil and Gas Technology Program
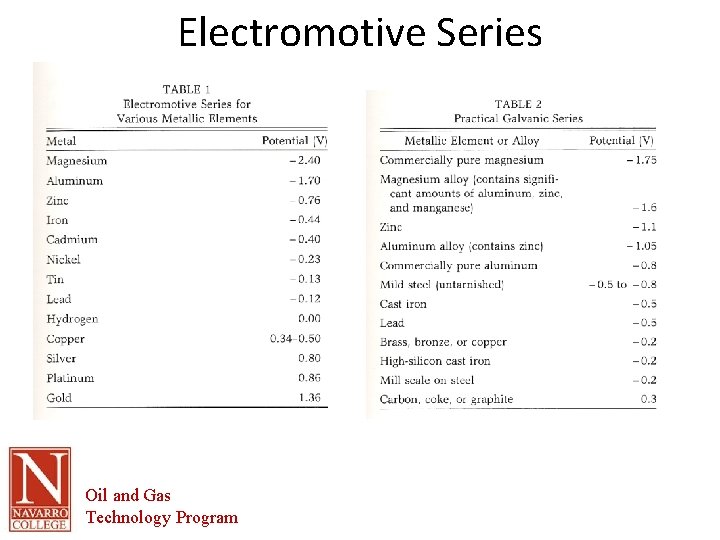
Electromotive Series Oil and Gas Technology Program
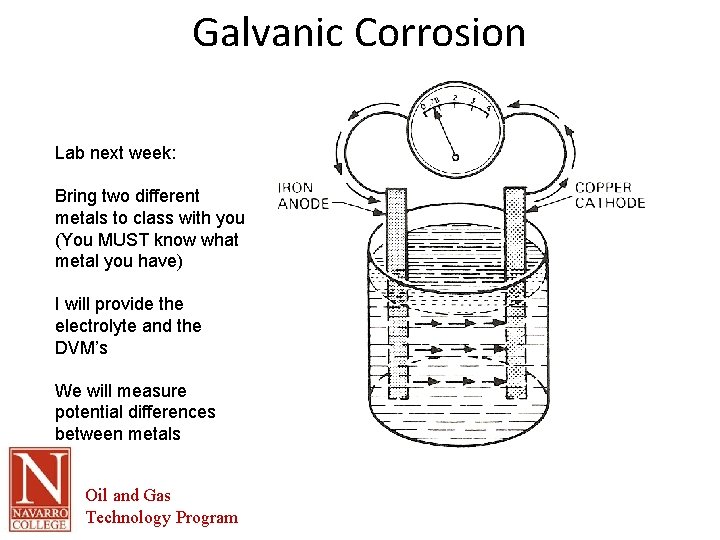
Galvanic Corrosion Lab next week: Bring two different metals to class with you (You MUST know what metal you have) I will provide the electrolyte and the DVM’s We will measure potential differences between metals Oil and Gas Technology Program

Oil and Gas Technology Program

This union did not provide enough isolation. Oil and Gas Technology Program

Stress (Fatigue) • The more stressed portions will be anodic. • Stress may be locked in as a result of manufacturing or from service conditions. • Stress corrosion is difficult to detect. • Some examples of stress: • Heat • Impacts • Bending/flexing Oil and Gas Technology Program

Stress • Heat (welding) stresses the metal See the crack Oil and Gas Technology Program
Stray Current • Stray currents may originate from direct-current distribution lines, substations, or street railway systems, etc. , and flow into a pipe system or other steel structure. • The corrosion resulting from stray currents (external sources) is similar to that from galvanic cells (which generate their own current) • In the electrolyte and at the metal-electrolyte interfaces, chemical and electrical reactions occur and are the same as those in the galvanic cell. • Soil and water characteristics affect the corrosion rate in the same manner as with galvanic-type corrosion. • However, stray current strengths may be much higher than those produced by galvanic cells and, as a consequence, corrosion may be much more rapid. • Stray currents are more likely to operate over long distances. Seeking the path of least resistance, the stray current from a foreign installation may travel along a pipeline causing severe corrosion where it leaves the line. • Knowing when stray currents are present becomes highly important when remedial measures are undertaken since a simple sacrificial anode system is likely to be ineffectual in preventing corrosion under such circumstances. Oil and Gas Technology Program
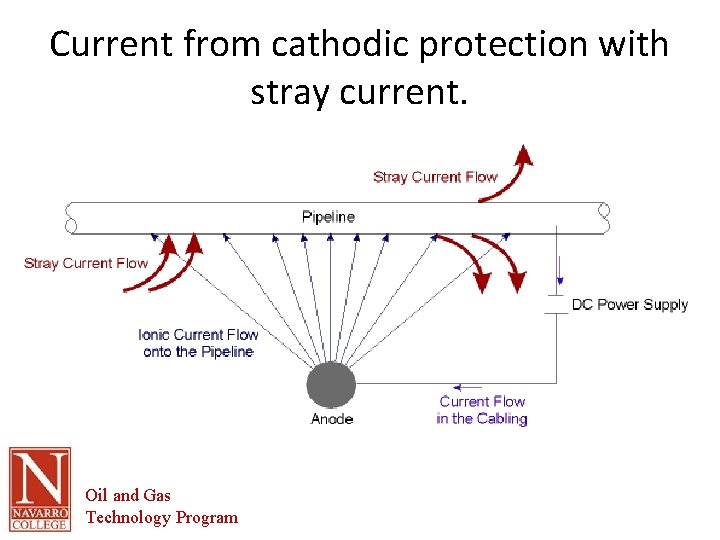
Current from cathodic protection with stray current. Oil and Gas Technology Program
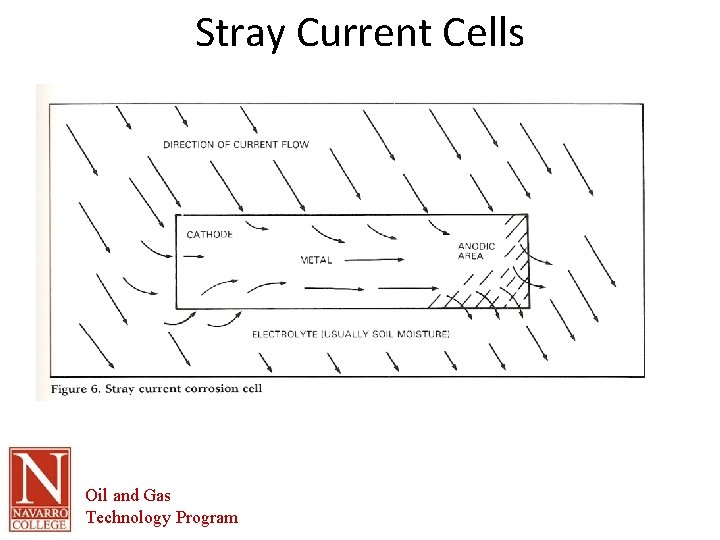
Stray Current Cells Oil and Gas Technology Program

Erosion • Erosion-corrosion arises from a combination of chemical attack and the physical abrasion as a consequence of the fluid motion • Surface deposits such as hydrogen can be removed, stimulating cell action. • Occurs frequently in bends, elbows, orifices and other areas where direction of flow changes or areas of turbulence. Oil and Gas Technology Program
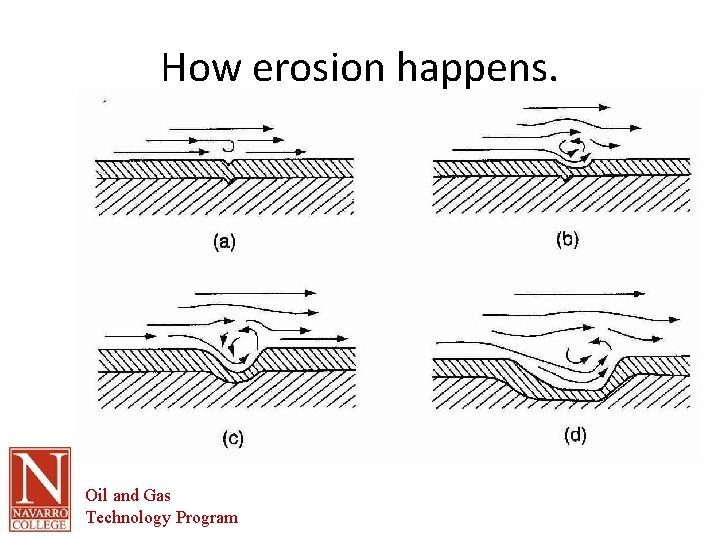
How erosion happens. Oil and Gas Technology Program

Erosion: What it looks like. Oil and Gas Technology Program

Nonuniform Surfaces • Non-uniform conditions along the surface of a metal can also cause different energy potentials. For example, the portion of an anchor embedded in concrete typically has lower energy potential than the portion exposed to soil. • New pipe inserted into existing pipe will be anodic due to the differences in the old pipe and new pipe. Oil and Gas Technology Program
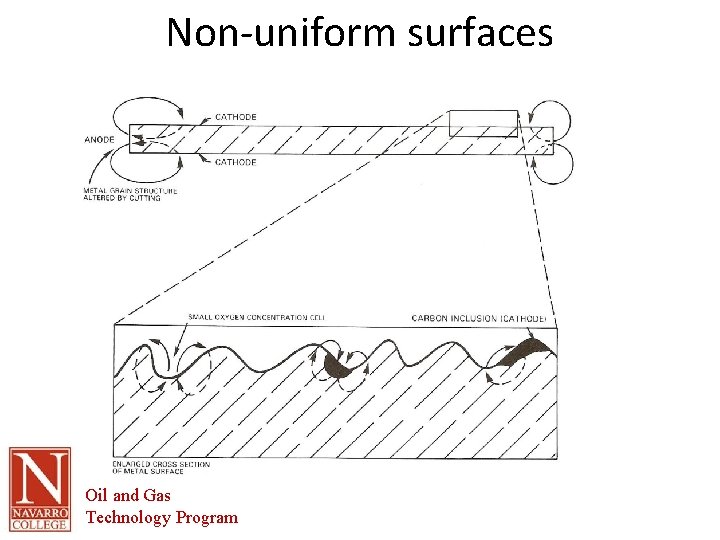
Non-uniform surfaces Oil and Gas Technology Program
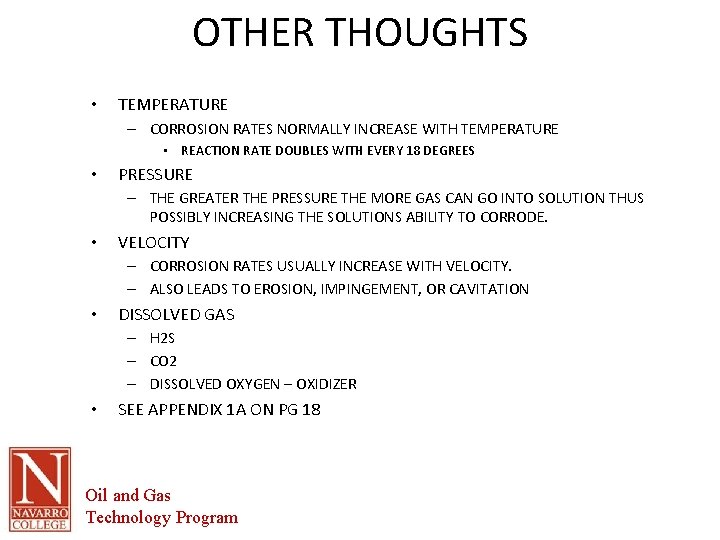
OTHER THOUGHTS • TEMPERATURE – CORROSION RATES NORMALLY INCREASE WITH TEMPERATURE • REACTION RATE DOUBLES WITH EVERY 18 DEGREES • PRESSURE – THE GREATER THE PRESSURE THE MORE GAS CAN GO INTO SOLUTION THUS POSSIBLY INCREASING THE SOLUTIONS ABILITY TO CORRODE. • VELOCITY – CORROSION RATES USUALLY INCREASE WITH VELOCITY. – ALSO LEADS TO EROSION, IMPINGEMENT, OR CAVITATION • DISSOLVED GAS – H 2 S – CO 2 – DISSOLVED OXYGEN – OXIDIZER • SEE APPENDIX 1 A ON PG 18 Oil and Gas Technology Program

Review • • • Corrosion costs the US $276, 000 each year Most of that corrosion is preventable Corrosion in the Oil and Gas Industry is both a financial issue as a safety issue Corrosion cell has 4 intrinsic parts Localized corrosion does not take long to render the asset “useless” Corrosion prevention in the oil and gas industry is required by law No water no corrosion Corrosion is an electrochemical reaction Shale plays and other resource development will bring corrosion issue to the forefront due to their prevalence and location All facets of this industry are effected by corrosion. Oil and Gas Technology Program
- Slides: 64