PSEUDOZEROORDER KINETICS DIFFUSION THROUGH A POLYMER MEMBRANE USING

PSEUDO-ZEROORDER KINETICS: DIFFUSION THROUGH A POLYMER MEMBRANE USING A SATURATED SOLUTION RESERVOIR SYSTEM Jan-alfred Aquino | Christopher Chen | Yanglu Chen | Zachariah De. Giulio | Katherine Dong | Christina Floristean | Michelle Guo| Alexandra Kapadia | Robert Kolchmeyer | Erik Massenzio | Adam Richardson | Jessica Xu Dr. David Cincotta | Alberto Rivera
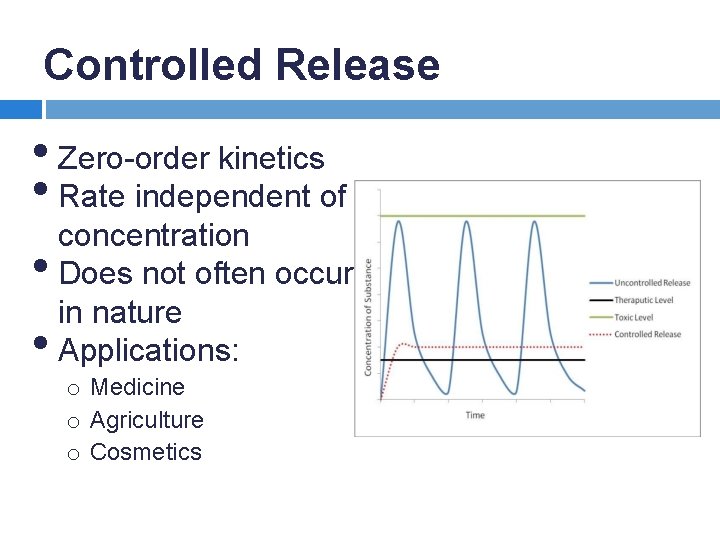
Controlled Release • Zero-order kinetics • Rate independent of concentration • Does not often occur in nature • Applications: o Medicine o Agriculture o Cosmetics

Pseudo-Zero-Order Kinetics Fick's Law: A pseudo-zero-order system would achieve a constant rate of release, but would not be independent of concentration. This can be done by holding the concentration gradient constant.

Hypothesis • IF: An apparatus is made that allows • • for diffusion of a saturated solution into a body of water across a membrane THEN: Pseudo-zero-order diffusion should be observed Maintain a constant concentration gradient
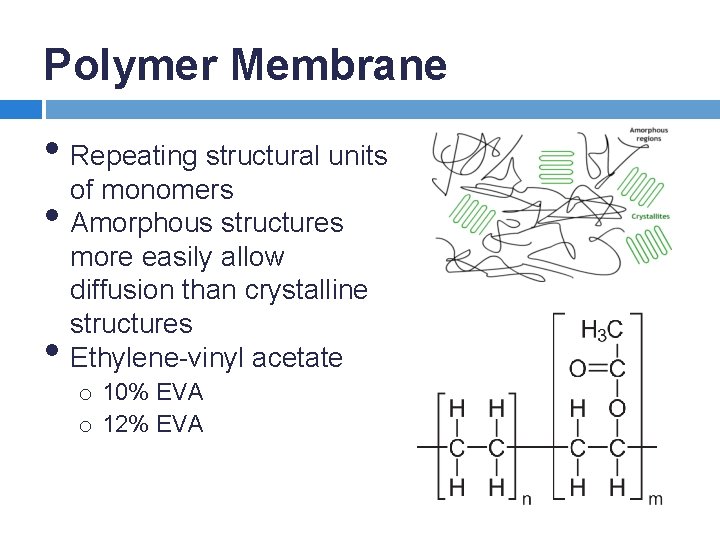
Polymer Membrane • Repeating structural units of monomers • Amorphous structures • more easily allow diffusion than crystalline structures Ethylene-vinyl acetate o 10% EVA o 12% EVA
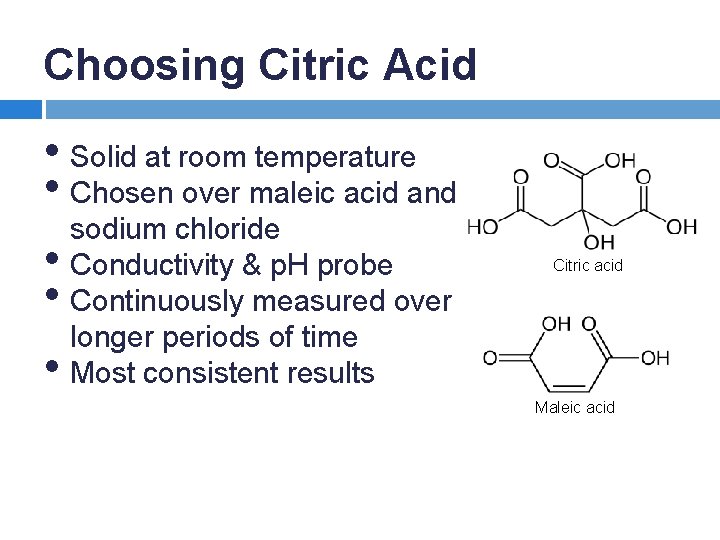
Choosing Citric Acid • Solid at room temperature • Chosen over maleic acid and sodium chloride • Conductivity & p. H probe • Continuously measured over longer periods of time • Most consistent results Citric acid Maleic acid
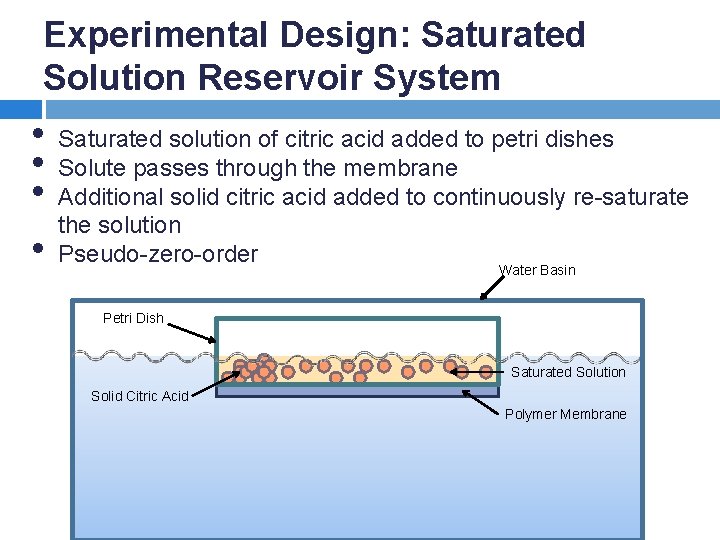
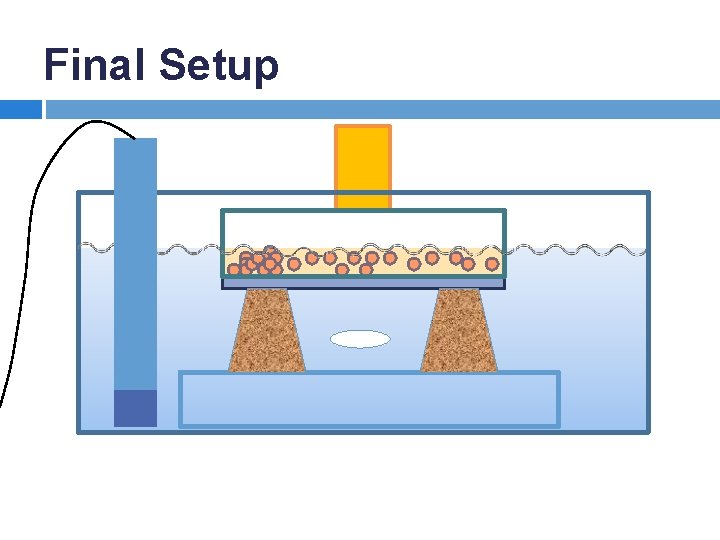
Experimental Design: Saturated Solution Reservoir System • • Saturated solution of citric acid added to petri dishes Solute passes through the membrane Additional solid citric acid added to continuously re-saturate the solution Pseudo-zero-order Water Basin Petri Dish Saturated Solution Solid Citric Acid Polymer Membrane
Final Setup

Experimental Design Features: Water-tight petri dishes Petri dish supports Maximize water and solution contact with the membrane Surface area optimization Uniform water level with respect to the petri dish • • •
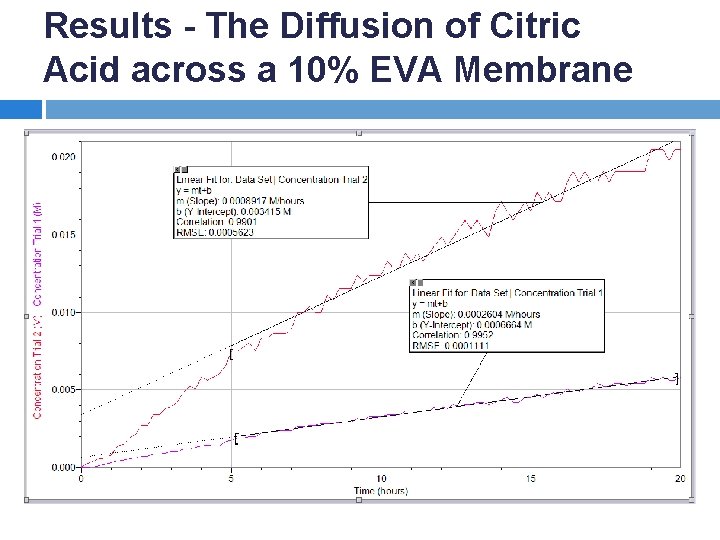
Results - The Diffusion of Citric Acid across a 10% EVA Membrane
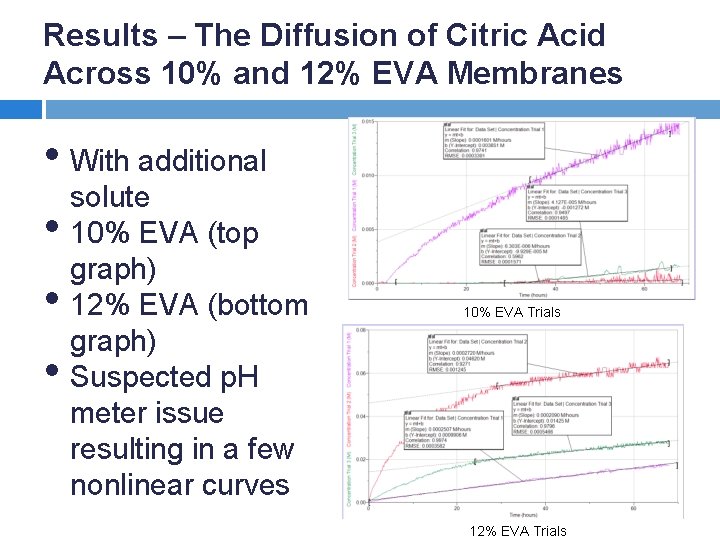
Results – The Diffusion of Citric Acid Across 10% and 12% EVA Membranes • With additional solute • 10% EVA (top graph) • 12% EVA (bottom graph) • Suspected p. H 10% EVA Trials meter issue resulting in a few nonlinear curves 12% EVA Trials
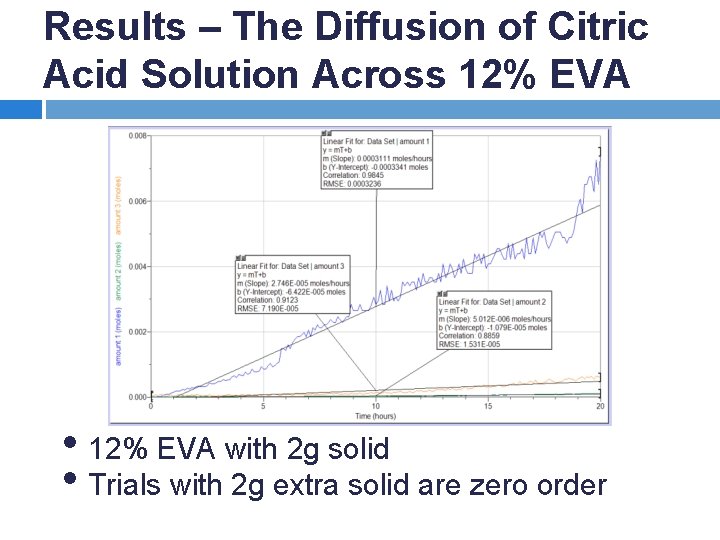
Results – The Diffusion of Citric Acid Solution Across 12% EVA • 12% EVA with 2 g solid • Trials with 2 g extra solid are zero order
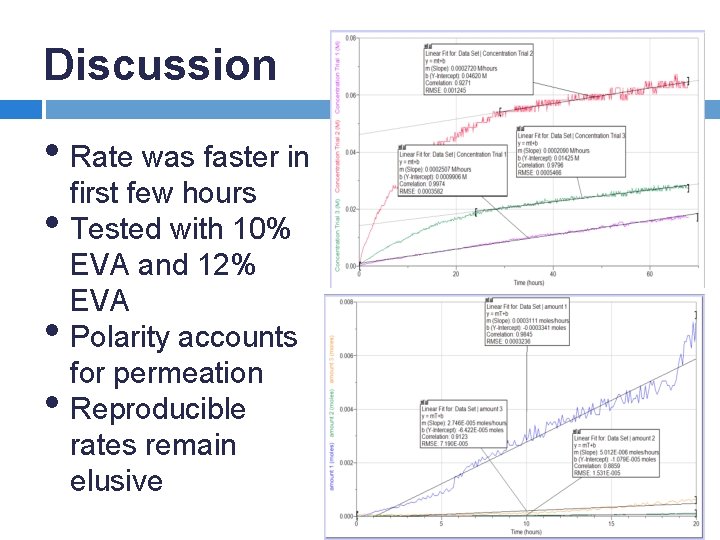
Discussion • Rate was faster in first few hours • Tested with 10% • • EVA and 12% EVA Polarity accounts for permeation Reproducible rates remain elusive
Conclusion • Achieved pseudo-zero-order kinetics with reservoir system • Polar likes polar: Citric acid • • • diffused through 12% EVA more effectively than 10% EVA Novel method for modeling a controlled-release system Data was supportive of hypothesis More needs to be done to conclusively accept or refute the hypothesis
Future Studies • Explore alternate methods of measuring diffusion • Solute embedded in polymer • Biodegradable polymers • Solute with lower solubility • Mathematical models based on • parameters of polymer membrane and solute Generalized curve fits

References 1. Prisciandaro M. and Pepe F. Absorption with zero and Pseudo-Zero order chemical reaction. The Canadian Journal of Chemical Engineering; (2006) [Internet] [accessed July 29, 2011] 362 – 368 p. . Available from : http: //onlinelibrary. wiley. com/doi/10. 1002/cjce. 5450750212/abstract 2. Nic M. , Jirat J. , Kosata B. Compendium of Chemical Terminology. IUPAC Goldbook; (2006). [Internet] [ accessed July 27, 2012] Available from : http: //goldbook. iupac. org/P 04937. html 3. Blackmond DG. , Hodnett NS. , Lloyd-Jones GC. , Mechanistic Implications of Pseudo Zero Order Kinetics in Kinetic Resolutions. JACS Communications; (2006). [Internet] [accessed July 29, 2011] 7450 - 7451 p. Available from : http: //pubs. acs. org/doi/pdfplus/10. 1021/ja 062173 f 4. Jones D. FASTtrack: Pharmaceutics: Dosage Form and Design. London: Pharmaceutical Press; (2008). [Internet] [accessed 2012] Available from : http: //www. pharmpress. com/files/docs/FT_Pharmaceutics_Drug_Delivery_sample. pdf 5. Basic Property of EVA Resin. SNE Research; (2006) [Internet] [accessed July 26, 2012] Available from : http: //www. sneresearch. com/eng/info/show. php? c_id=4938&pg=6&s_sort=&sub_cat=&s_type=&s_word= 6. Ionization Constants of Heteroatom Organic Acids. Michigan State University. [Internet] [accessed July 26, 2012] Available from : http: //www 2. chemistry. msu. edu/faculty/reusch/Virt. Txt. Jml/acidity 2. htm 7. Material Safety Data Sheet - Citric Acid. Science Lab. [Internet] [accessed July 24, 2012] Available from : http: //www. sciencelab. com/msds. php? msds. Id=9923495 8. Periodontics: Controlled release delivery system. British Dental Journal; (2003). [Internet] [accessed July 30, 2012] 195 - 224 p. Available from : http: //www. nature. com/bdj/journal/v 195/n 4/full/4810497 a. html 9. Controlled release of triprolidine using ethylene-vinyl acetate membrane and matrix systems. European Journal Pharmaceutics and Biopharmaceutics. (2002) [Internet] [accessed 2012] 201 - 206 p. Available from : http: //www. sciencedirect. com. ezproxy. drew. edu/science/article/pii/S 0939641102000516 10. Gupta C. , Chauhan A. Drug transport in HEMA conjuctival inserts containing precipitated drug particles. Journal of Colloid and Interface Science ; (2010). [Internet] [accessed July 30, 2012] 31 - 42 p. Available from : http: //www. sciencedirect. com. ezproxy. drew. edu/science/article/pii/S 0021979710003267 11. Drabczyk K. , Panek Piotr. A Comparative Study of EVA with and without thermal history for different lamination process parameters. Material Sciences and Engineering: B; (2011). [Internet] [accessed July 29, 2012] Available from : http: //www. sciencedirect. com/science/article/pii/S 0921510712002541 12. Krishnan KA. , Sreejalekshmi KG. , Varghese S. Adsorptive Retention of Citric Acid Onto Activated Carbon Prepared From Havea Braziliansis Sawdust: Kinetic and Isotherm Overview. Desalination; (2010). [Internet] [accessed July 30, 2012] 46 - 52 p. Available from : http: //www. sciencedirect. com/science/article/pii/S 001191641000144 X

Acknowledgements Dr. David Cincotta, Advisor Alberto Rivera, Assistant Dr. David Miyamoto, Director New Jersey Governor's School in the Sciences and its Sponsors
- Slides: 17