Protons Neutrons ElectronsIsotopesAverage Atomic Mass An Atom is






- Slides: 21

Protons, Neutrons, Electrons/Isotopes/Average Atomic Mass

An Atom is Like… If an atom was the size of a football stadium … Gnat-sized electron • the nucleus (protons + neutrons) would be a marble on the 50 yard line • the electrons would be smaller than gnats out in the stands Marble -sized nucleus

Atom Facts! Important things to remember about atoms! Most of an atom is empty space Electrons are so small, we can pretend that their mass is zero The mass of an atom is from its nucleus (protons & neutrons)

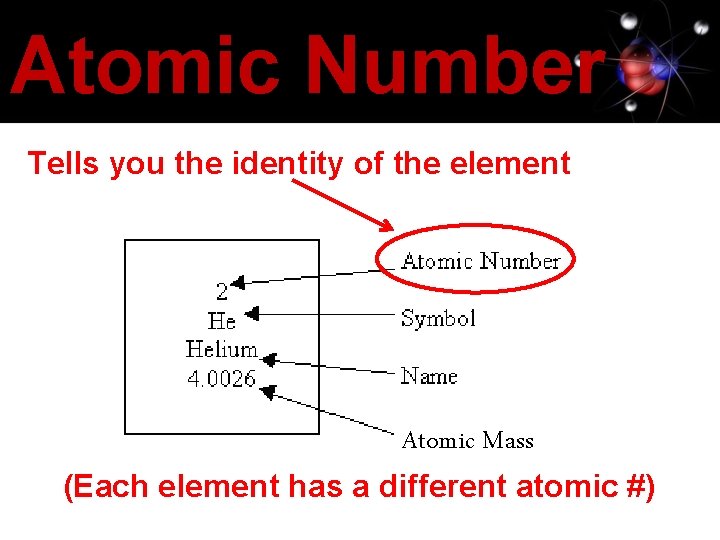
Atomic Number Tells you the identity of the element Atomic Mass (Each element has a different atomic #)

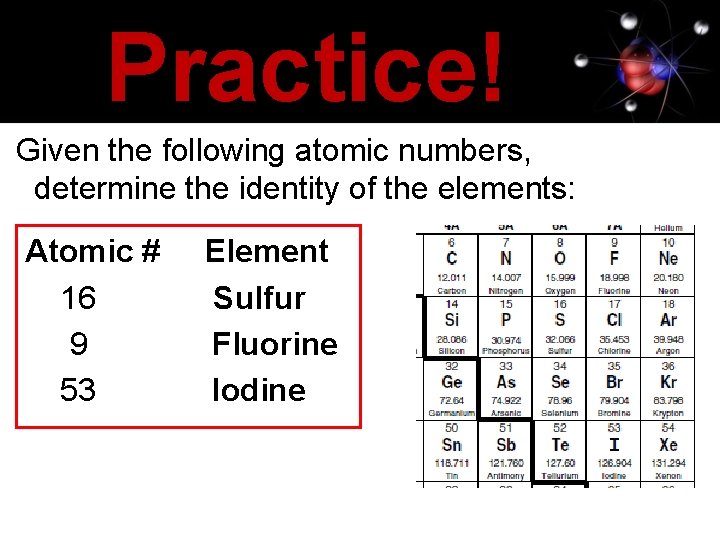
Practice! Given the following atomic numbers, determine the identity of the elements: Atomic # 16 9 53 Element Sulfur Fluorine Iodine

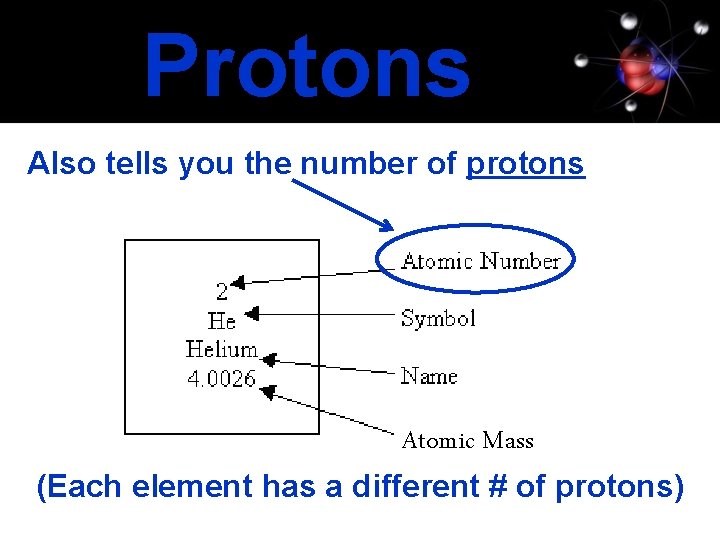
Protons Also tells you the number of protons Atomic Mass (Each element has a different # of protons)

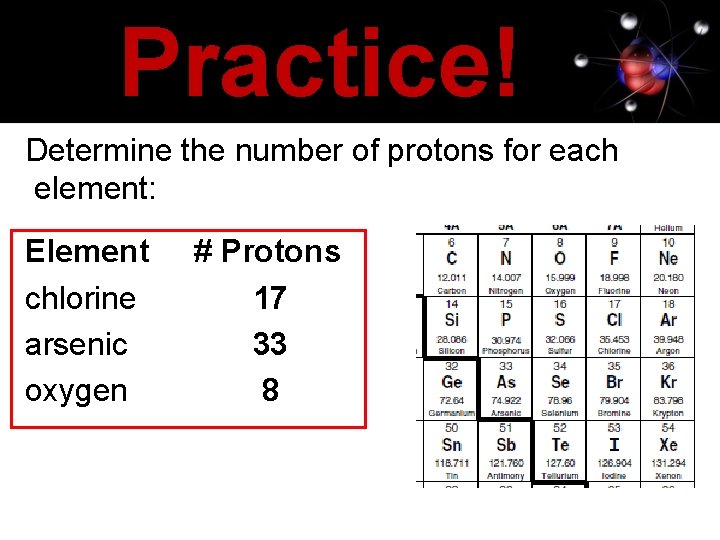
Practice! Determine the number of protons for each element: Element chlorine arsenic oxygen # Protons 17 33 8

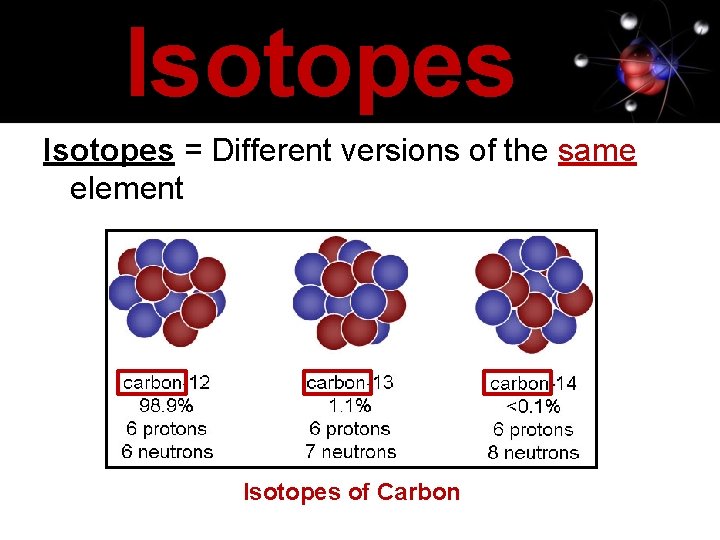
Isotopes = Different versions of the same element Isotopes of Carbon

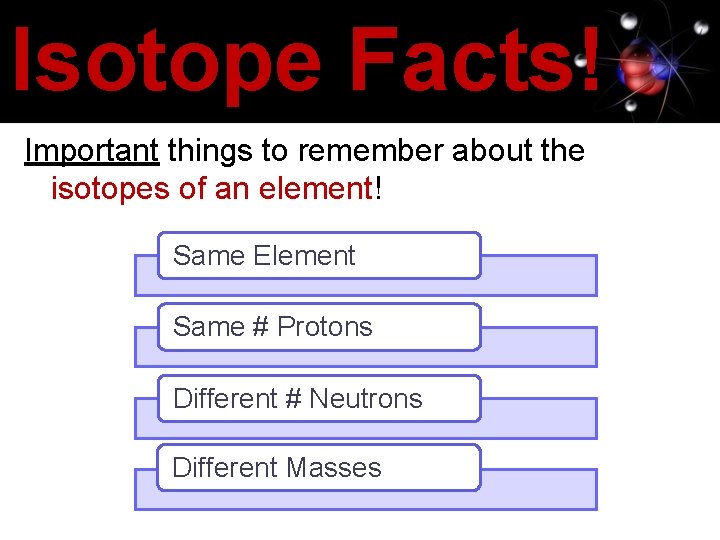
Isotope Facts! Important things to remember about the isotopes of an element! Same Element Same # Protons Different # Neutrons Different Masses

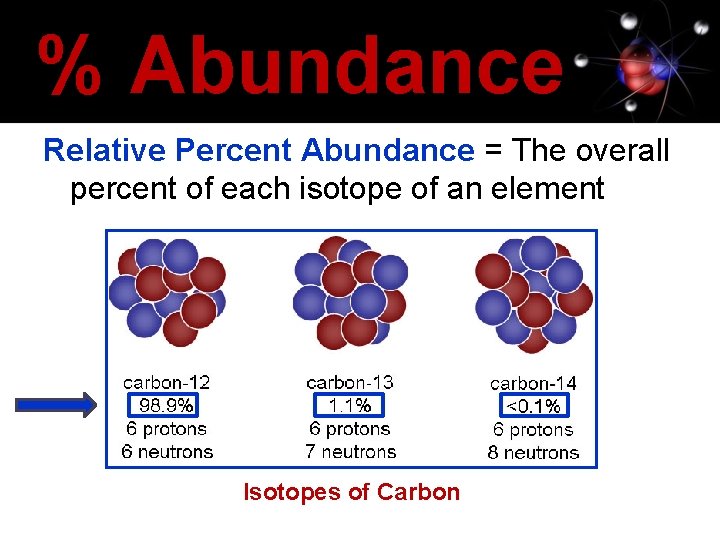
% Abundance Relative Percent Abundance = The overall percent of each isotope of an element Isotopes of Carbon

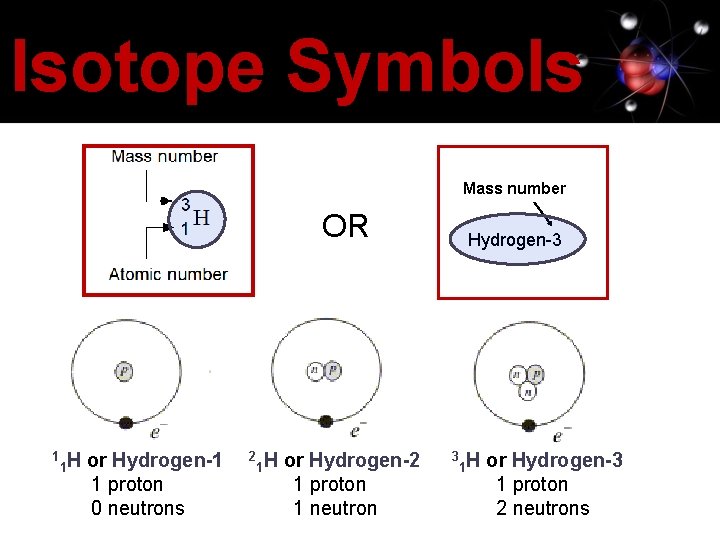
Isotope Symbols Mass number OR 1 1 H or Hydrogen-1 1 proton 0 neutrons 2 1 H or Hydrogen-2 1 proton 1 neutron Hydrogen-3 3 1 H or Hydrogen-3 1 proton 2 neutrons

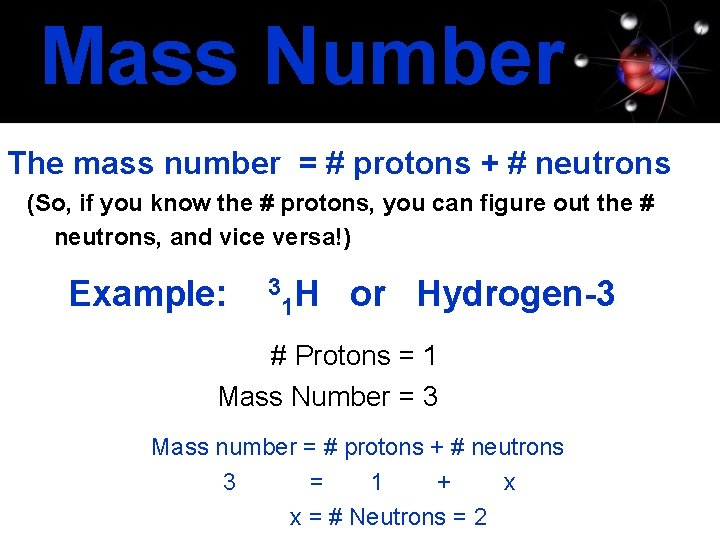
Mass Number The mass number = # protons + # neutrons (So, if you know the # protons, you can figure out the # neutrons, and vice versa!) Example: 3 1 H or Hydrogen-3 # Protons = 1 Mass Number = 3 Mass number = # protons + # neutrons 3 = 1 + x x = # Neutrons = 2

Practice! Determine the # of protons and neutrons for each of the following isotopes: 16 8 O 8 protons, 8 neutrons 21 10 Ne 10 protons, 11 neutrons Flourine-19 9 protons, 10 neutrons Boron-11 5 protons, 6 neutrons

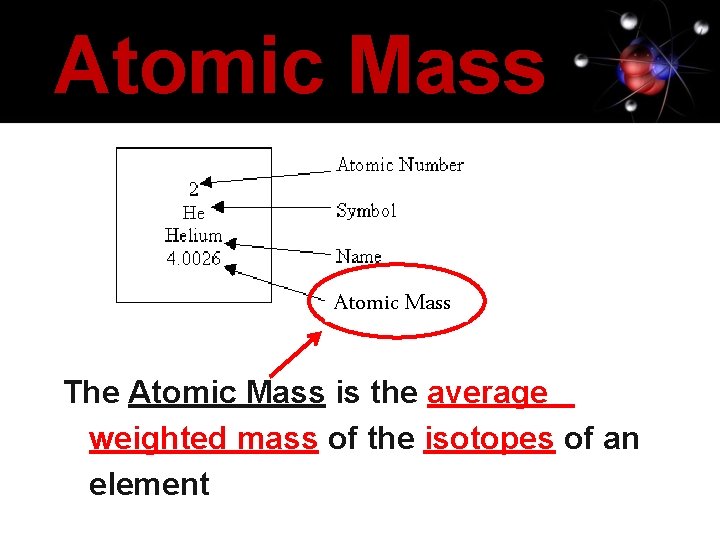
Atomic Mass The Atomic Mass is the average weighted mass of the isotopes of an element

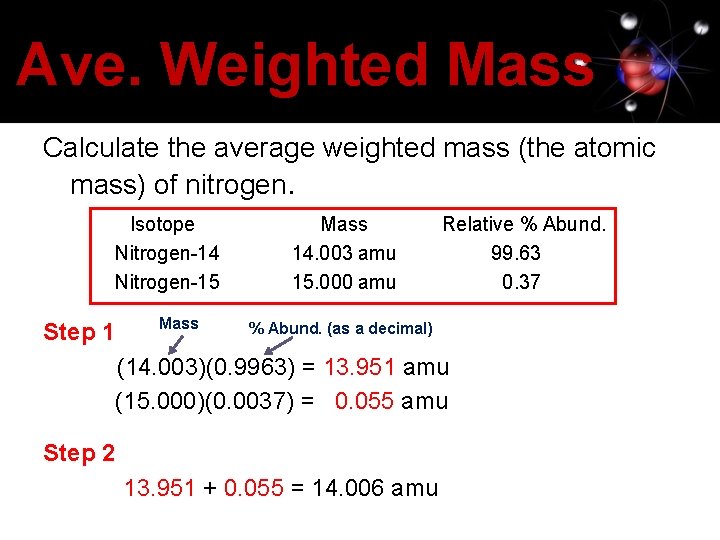
Ave. Weighted Mass To calculate the average weighted mass (atomic mass of an element: Step 1 Multiply each isotope’s mass x its % abundance. (Make sure the % abundance is in decimal form!) Step 2 Add all of the answers from step 1 above.

Ave. Weighted Mass Calculate the average weighted mass (the atomic mass) of nitrogen. Isotope Nitrogen-14 Nitrogen-15 Step 1 Mass 14. 003 amu 15. 000 amu Relative % Abund. 99. 63 0. 37 % Abund. (as a decimal) (14. 003)(0. 9963) = 13. 951 amu (15. 000)(0. 0037) = 0. 055 amu Step 2 13. 951 + 0. 055 = 14. 006 amu

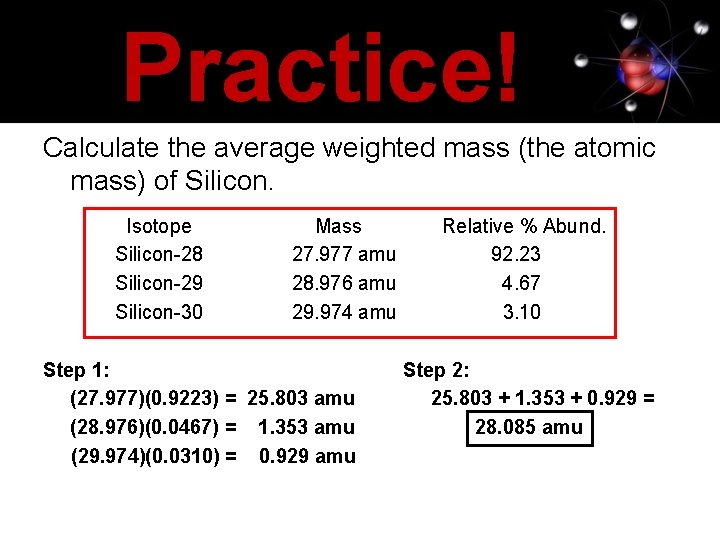
Practice! Calculate the average weighted mass (the atomic mass) of Silicon. Isotope Silicon-28 Silicon-29 Silicon-30 Mass 27. 977 amu 28. 976 amu 29. 974 amu Step 1: (27. 977)(0. 9223) = 25. 803 amu (28. 976)(0. 0467) = 1. 353 amu (29. 974)(0. 0310) = 0. 929 amu Relative % Abund. 92. 23 4. 67 3. 10 Step 2: 25. 803 + 1. 353 + 0. 929 = 28. 085 amu

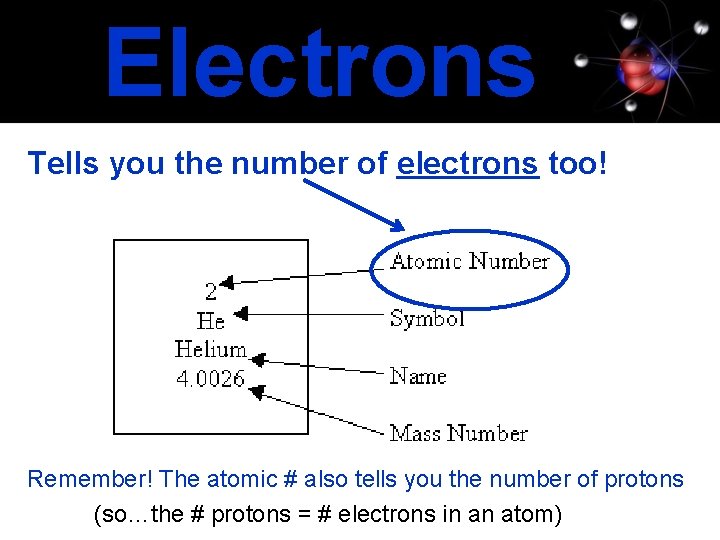
Electrons Tells you the number of electrons too! Remember! The atomic # also tells you the number of protons (so…the # protons = # electrons in an atom)

Ions! When an atom loses or gains one or more electrons, it becomes an ion. There are two kinds of ions: • Cations: Ions formed when atoms lose one or more electrons – have a positive charge Ex. Ca 2+, Na 1+, Al 3+ • Anions: Ions formed when atoms gain one or more electrons – have a negative charge Ex. F 1 -, P 3 -, O 2 -

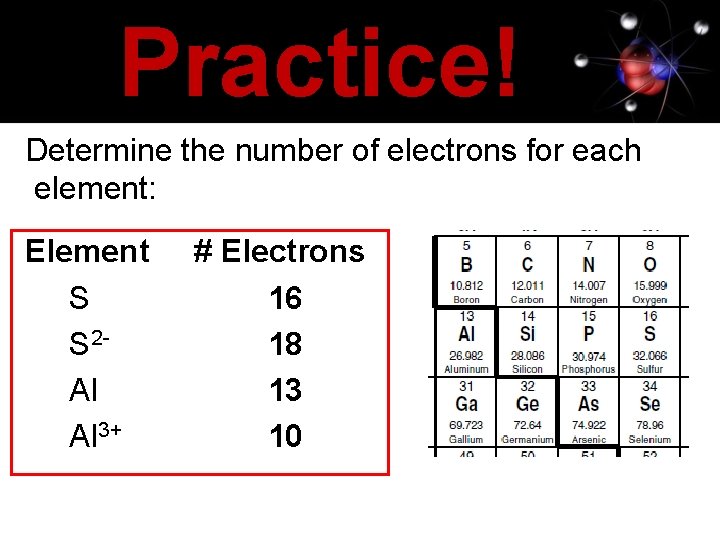
Practice! Determine the number of electrons for each element: Element S S 2 Al Al 3+ # Electrons 16 18 13 10

Finished!