Proton Sponges A Rigid Organic Scaffold to Reveal

- Slides: 19

Proton Sponges: A Rigid Organic Scaffold to Reveal the Quantum Structure of the Intramolecular Proton Bond 1 8 Andrew F. De. Blase, Michael T. Scerba, Thomas Lectka, and Mark A. Johnson June 22, 2012

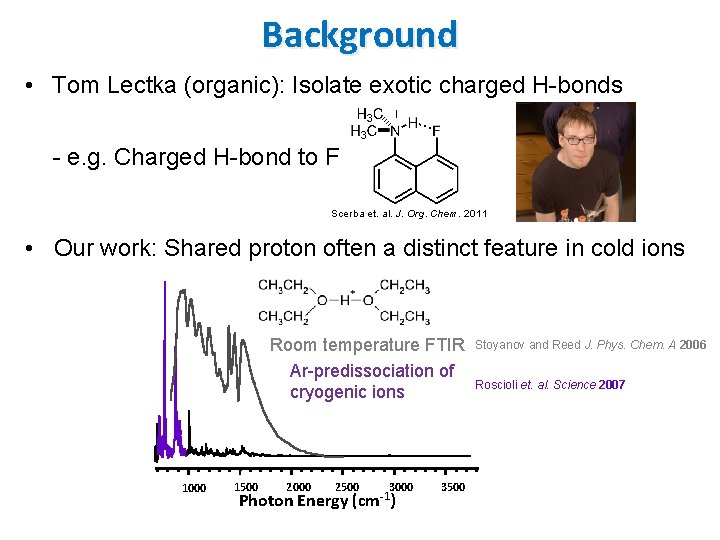
Background • Tom Lectka (organic): Isolate exotic charged H-bonds - e. g. Charged H-bond to F Scerba et. al. J. Org. Chem. 2011 • Our work: Shared proton often a distinct feature in cold ions Room temperature FTIR Ar-predissociation of cryogenic ions 1000 1500 2000 2500 Photon Energy 3000 -1 (cm ) 3500 Stoyanov and Reed J. Phys. Chem. A 2006 Roscioli et. al. Science 2007

Background • Tom Lectka (organic): Isolate exotic charged H-bonds - e. g. Charged H-bond to F Scerba et. al. J. Org. Chem. 2011 • Our work: Shared proton often a distinct feature in cold ions “I think this is the beginning of a beautiful friendship. ”

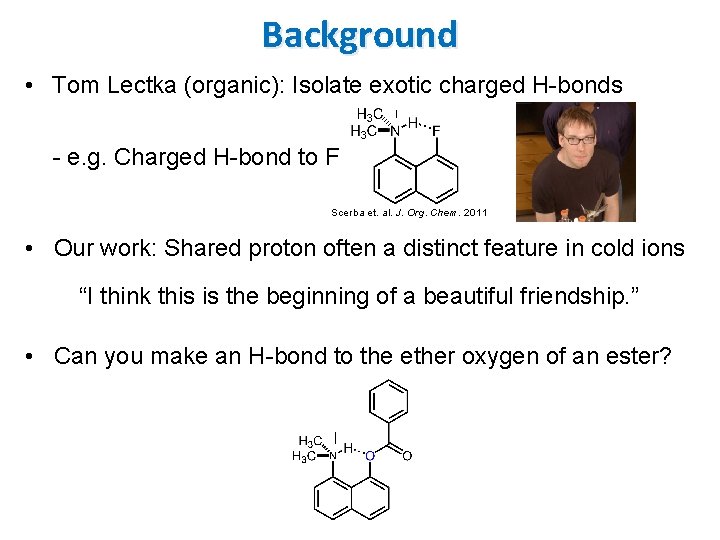
Background • Tom Lectka (organic): Isolate exotic charged H-bonds - e. g. Charged H-bond to F Scerba et. al. J. Org. Chem. 2011 • Our work: Shared proton often a distinct feature in cold ions “I think this is the beginning of a beautiful friendship. ” • Can you make an H-bond to the ether oxygen of an ester?

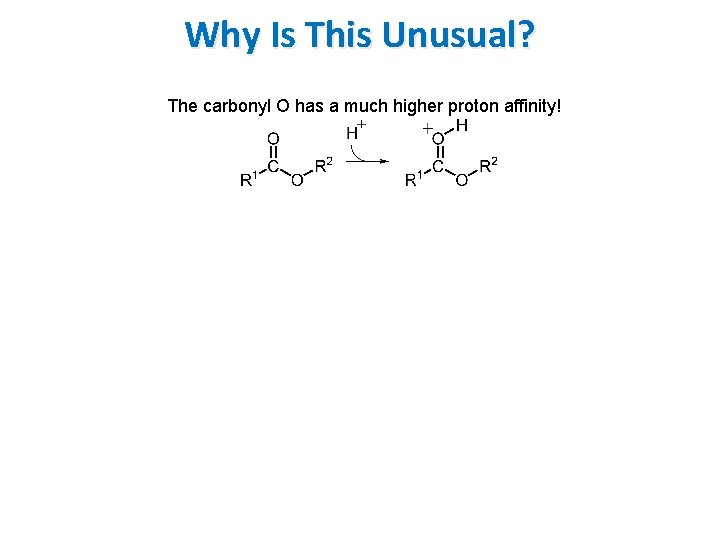
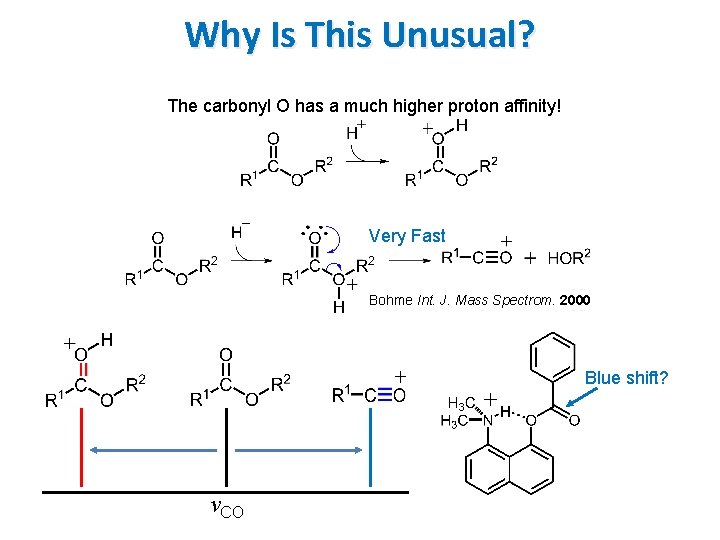
Why Is This Unusual? The carbonyl O has a much higher proton affinity!

Why Is This Unusual? The carbonyl O has a much higher proton affinity! Very Fast Bohme Int. J. Mass Spectrom. 2000 Blue shift? νCO

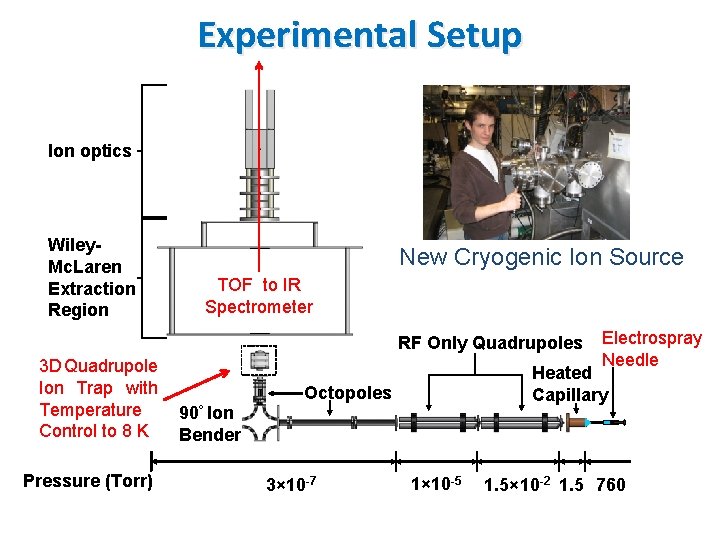
Experimental Setup Ion optics Wiley. Mc. Laren Extraction Region New Cryogenic Ion Source TOF to IR Spectrometer RF Only Quadrupoles 3 D Quadrupole Ion Trap with Temperature Control to 8 K Pressure (Torr) Heated Capillary Octopoles 90° Ion Bender 3× 10 -7 Electrospray Needle 1× 10 -5 1. 5× 10 -2 1. 5 760

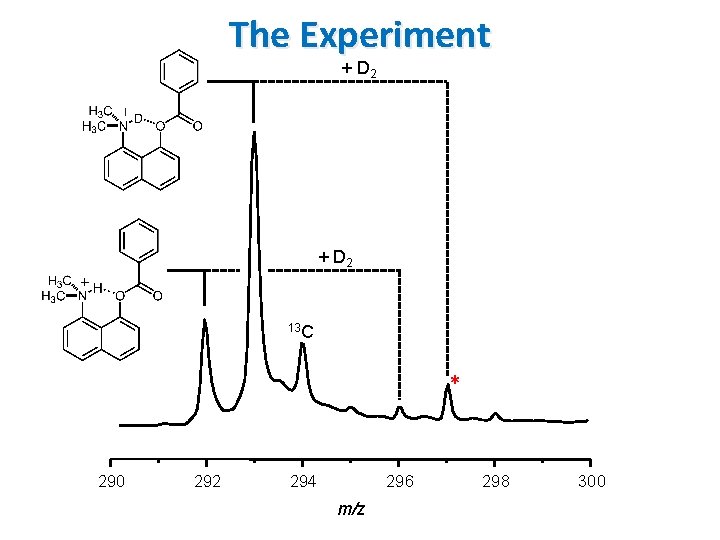
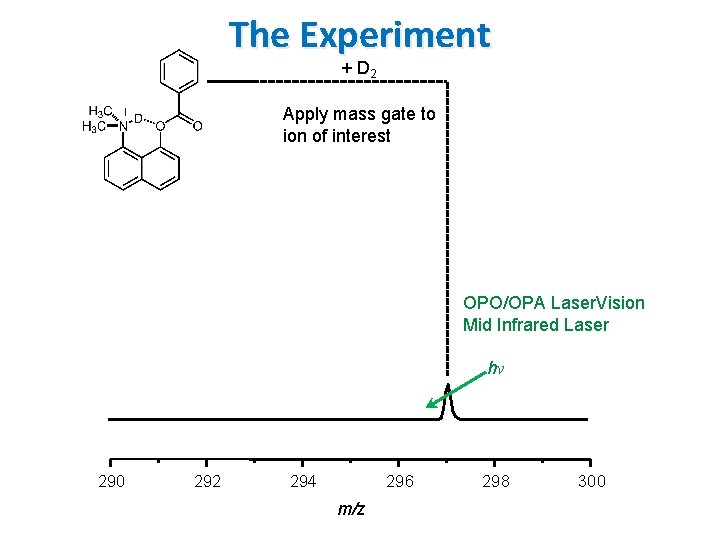
The Experiment + D 2 13 C * 290 292 294 296 m/z 298 300

The Experiment + D 2 Apply mass gate to ion of interest OPO/OPA Laser. Vision Mid Infrared Laser 13 C hν 290 292 294 296 m/z 298 300

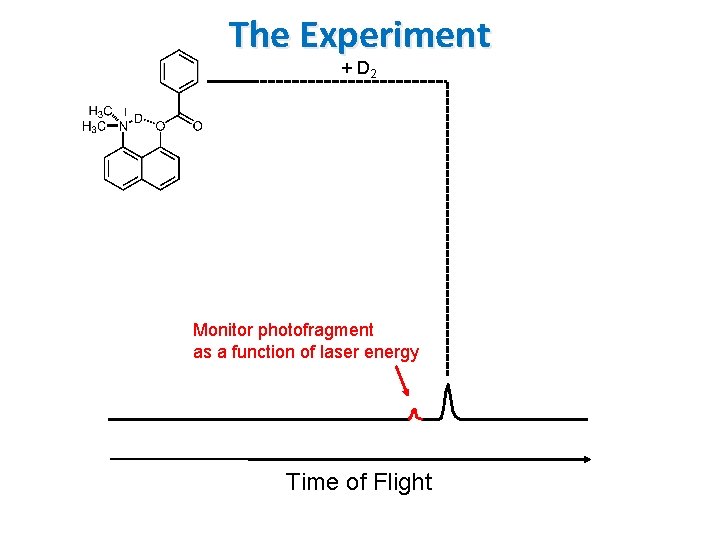
The Experiment + D 2 13 C Monitor photofragment as a function of laser energy Time of Flight

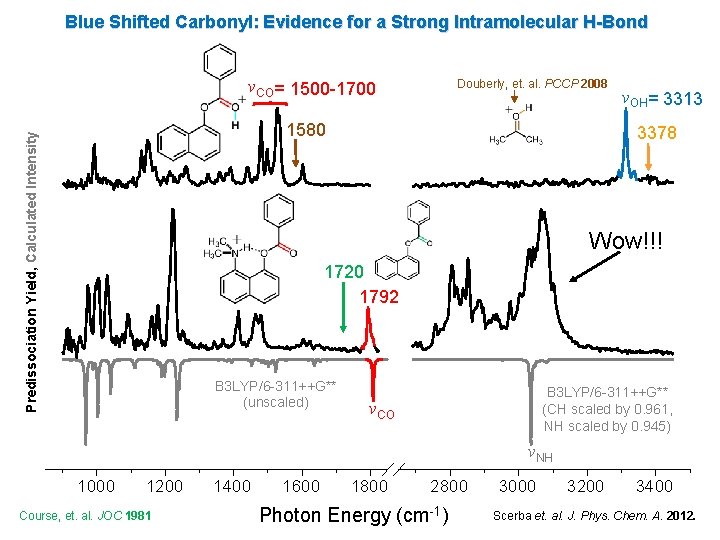
Blue Shifted Carbonyl: Evidence for a Strong Intramolecular H-Bond νCO= 1500 -1700 Douberly, et. al. PCCP 2008 Predissociation Yield, Calculated Intensity 1580 νOH= 3313 3378 Wow!!! 1720 1792 B 3 LYP/6 -311++G** (unscaled) B 3 LYP/6 -311++G** (CH scaled by 0. 961, NH scaled by 0. 945) νCO νNH 1000 1200 Course, et. al. JOC 1981 1400 1600 1800 2800 Photon Energy (cm-1) 3000 3200 3400 Scerba et. al. J. Phys. Chem. A. 2012.

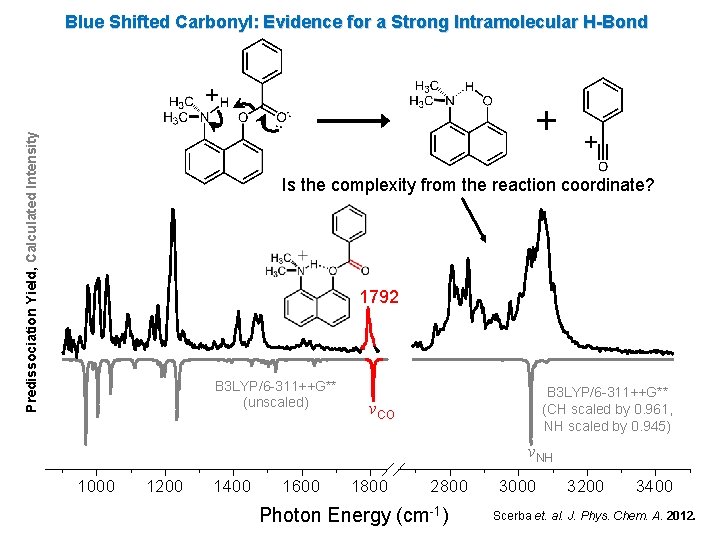
Blue Shifted Carbonyl: Evidence for a Strong Intramolecular H-Bond Predissociation Yield, Calculated Intensity + + + Is the complexity from the reaction coordinate? 1792 B 3 LYP/6 -311++G** (unscaled) B 3 LYP/6 -311++G** (CH scaled by 0. 961, NH scaled by 0. 945) νCO νNH 1000 1200 1400 1600 1800 2800 Photon Energy (cm-1) 3000 3200 3400 Scerba et. al. J. Phys. Chem. A. 2012.

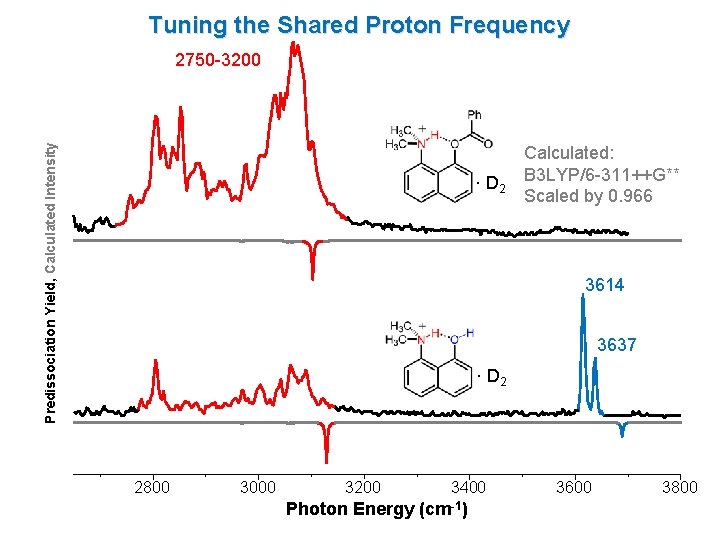
Tuning the Shared Proton Frequency Predissociation Yield, Calculated Intensity 2750 -3200 · D 2 Calculated: B 3 LYP/6 -311++G** Scaled by 0. 966 3614 3637 · D 2 2800 3000 3200 3400 Photon Energy (cm-1) 3600 3800

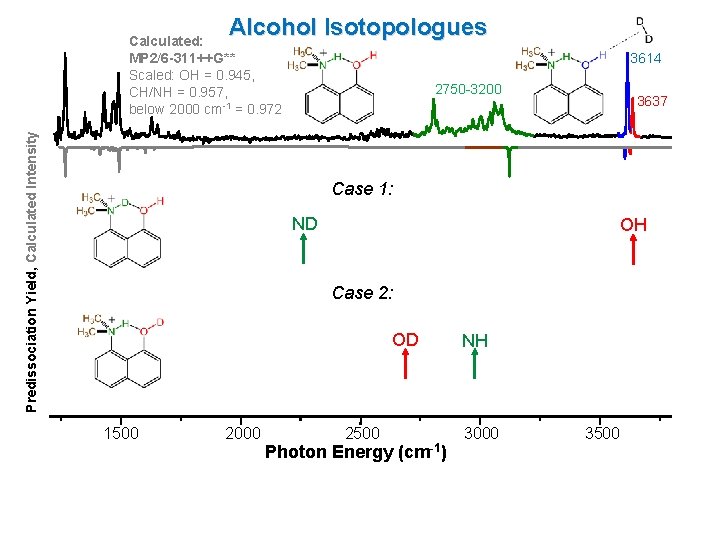
Alcohol Isotopologues Predissociation Yield, Calculated Intensity Calculated: MP 2/6 -311++G** Scaled: OH = 0. 945, CH/NH = 0. 957, below 2000 cm-1 = 0. 972 3614 2750 -3200 3637 Case 1: ND OH Case 2: OD 1500 2000 2500 Photon Energy (cm-1) NH 3000 3500

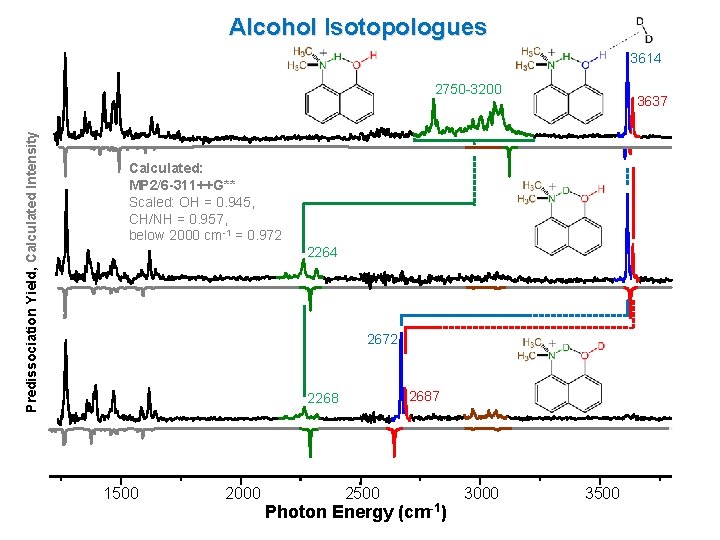
Alcohol Isotopologues 3614 Predissociation Yield, Calculated Intensity 2750 -3200 3637 Calculated: MP 2/6 -311++G** Scaled: OH = 0. 945, CH/NH = 0. 957, below 2000 cm-1 = 0. 972 2264 2672 2687 2268 1500 2000 2500 Photon Energy (cm-1) 3000 3500

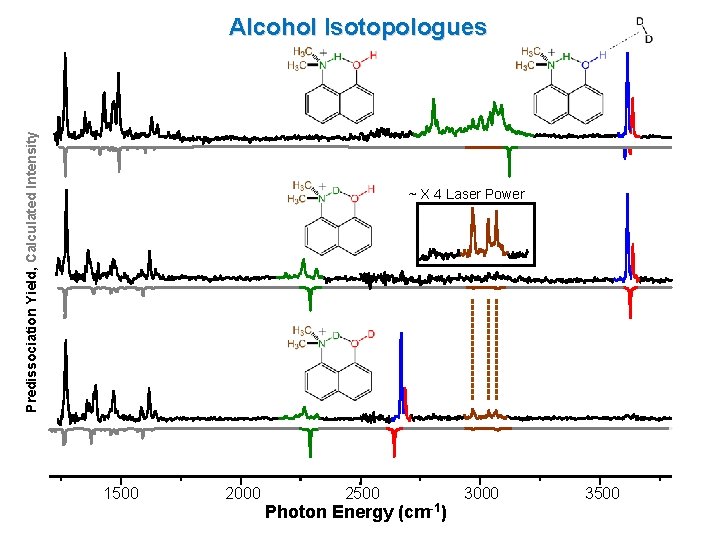
Predissociation Yield, Calculated Intensity Alcohol Isotopologues ~ X 4 Laser Power 1500 2000 2500 Photon Energy (cm-1) 3000 3500

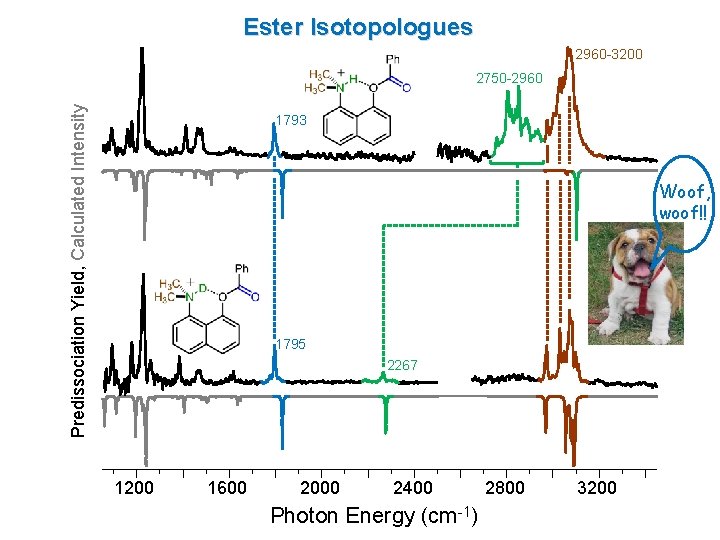
Ester Isotopologues 2960 -3200 Predissociation Yield, Calculated Intensity 2750 -2960 1793 Woof, woof!! 1795 2267 1200 1600 2000 2400 Photon Energy (cm-1) 2800 3200

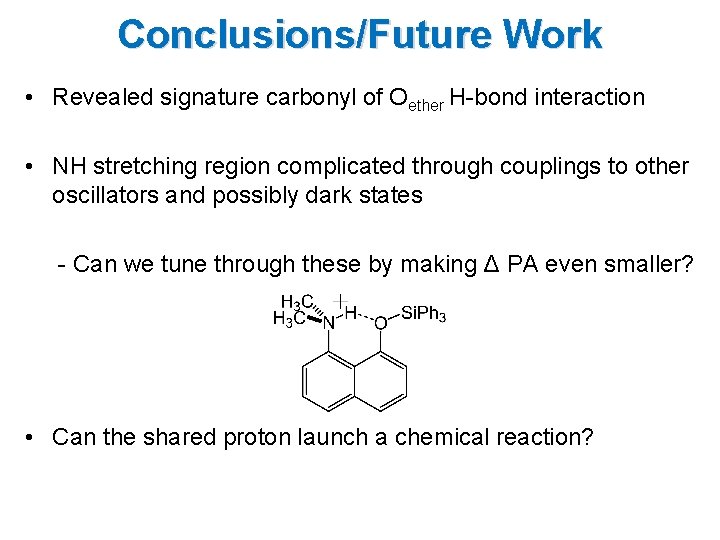
Conclusions/Future Work • Revealed signature carbonyl of Oether H-bond interaction • NH stretching region complicated through couplings to other oscillators and possibly dark states - Can we tune through these by making Δ PA even smaller? • Can the shared proton launch a chemical reaction?

Acknowledgements • • • Labmates: Past and Current Mark: New science, new hobbies! Tom Lectka’s group at JHU for making the molecules! Ken Jordan for help with calculations Anne Mc. Coy for useful conversations about anharmonicity Funding: National Science Foundation, Air Force