PROTON PUMP INHIBITORS Medicinal Chemistry III B Pharm
PROTON PUMP INHIBITORS Medicinal Chemistry - III B. Pharm. Dr Pran Kishore Deb and Dr. Bilal Al-Jaidi

Learning Outcomes At the end of the lecture students will be able to Ø Understand where and how drugs work Ø Understand principles of how drugs are designed Ø Understand process of how drugs are designed Ø Illustrate drug design and development of proton pump inhibitors 10/22/2021 using examples 2
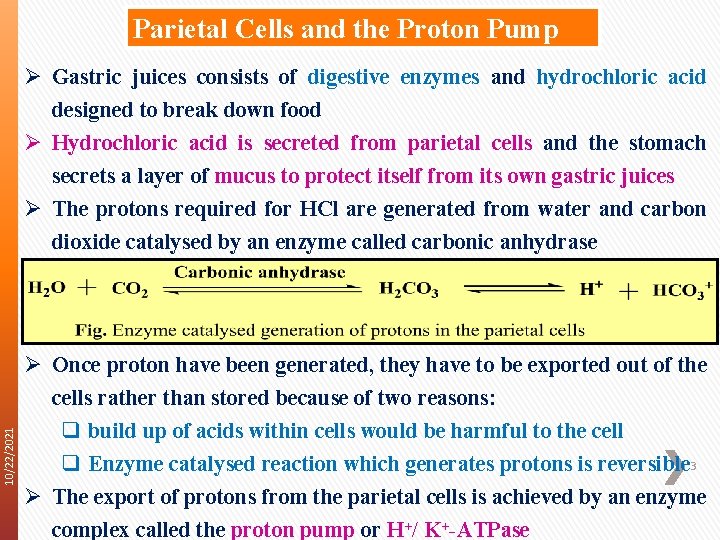
Parietal Cells and the Proton Pump 10/22/2021 Ø Gastric juices consists of digestive enzymes and hydrochloric acid designed to break down food Ø Hydrochloric acid is secreted from parietal cells and the stomach secrets a layer of mucus to protect itself from its own gastric juices Ø The protons required for HCl are generated from water and carbon dioxide catalysed by an enzyme called carbonic anhydrase Ø Once proton have been generated, they have to be exported out of the cells rather than stored because of two reasons: q build up of acids within cells would be harmful to the cell q Enzyme catalysed reaction which generates protons is reversible 3 Ø The export of protons from the parietal cells is achieved by an enzyme complex called the proton pump or H+/ K+-ATPase

Parietal Cells and the Proton Pump Histamine Acetylcholine M 3 H 2 ATP Receptors Proton pump H+ K+ Cl- 10/22/2021 Cck 2 ADP + Pi Parietal cell Canaliculus Gastrin HCl Ion channels Lumen of the stomach Ø When the parietal cells are actively secreting HCl into the stomach, they form invaginations called canaliculus. Ø Proton pump is present in the canalicular membrane of parietal cells Ø Pumps protons out of the parietal cell and potassium ions back in 4 Ø Requires energy - provided by hydrolysis of ATP to ADP, catalysed by ATPase Ø The proton pump is also called H+/K+-ATPase

Parietal Cells and the Proton Pump 10/22/2021 Ø Ø Proton pump is not responsible for the efflux of chloride ion Chloride ions depart through a separate ion channel HCl is formed in the canaliculus The potassium ions exit the parietal cell as counterions for the chloride ions and are then pumped back in Ø A separate potassium ion channel is used for K+ ions leaving the cell 5
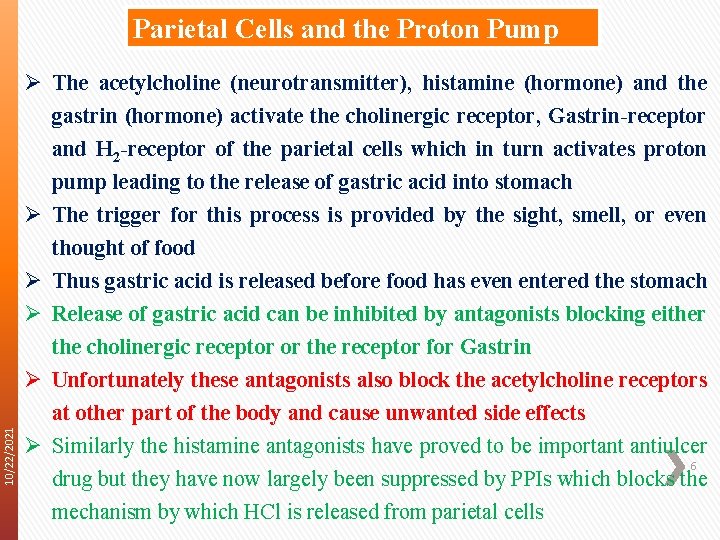
10/22/2021 Parietal Cells and the Proton Pump Ø The acetylcholine (neurotransmitter), histamine (hormone) and the gastrin (hormone) activate the cholinergic receptor, Gastrin-receptor and H 2 -receptor of the parietal cells which in turn activates proton pump leading to the release of gastric acid into stomach Ø The trigger for this process is provided by the sight, smell, or even thought of food Ø Thus gastric acid is released before food has even entered the stomach Ø Release of gastric acid can be inhibited by antagonists blocking either the cholinergic receptor or the receptor for Gastrin Ø Unfortunately these antagonists also block the acetylcholine receptors at other part of the body and cause unwanted side effects Ø Similarly the histamine antagonists have proved to be important antiulcer 6 drug but they have now largely been suppressed by PPIs which blocks the mechanism by which HCl is released from parietal cells

Design and Development of Proton Pump Inhibitors Ø It was seen by Swedish scientists that local anaestetics related to lignocaine reduce gastric acid secretion in man when taken orally Ø Then the Swedish scientists attended a conference where they discovered that a potential antiviral drug called pyridylthioacetamide slowed down gastric secretion as a side effect 10/22/2021 Ø This drug was toxic due to thioacetate group Ø To overcome this other S-C-N groups were investigated and H 77/67 was discovered which had antisecretory activity 7
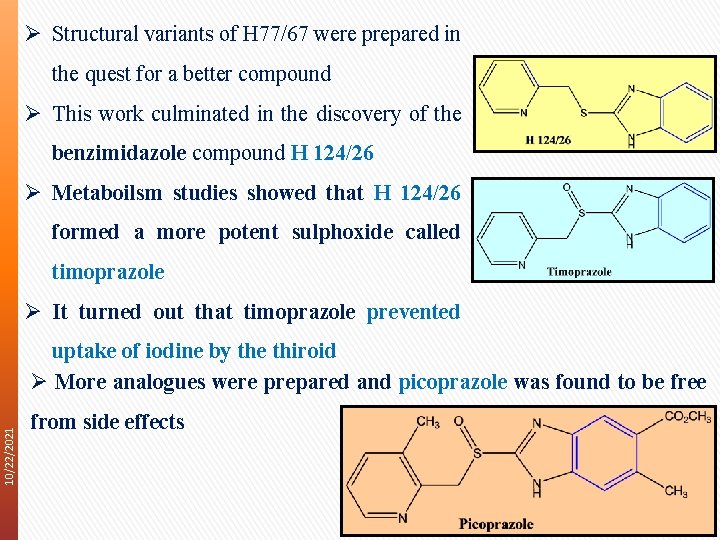
Ø Structural variants of H 77/67 were prepared in the quest for a better compound Ø This work culminated in the discovery of the benzimidazole compound H 124/26 Ø Metaboilsm studies showed that H 124/26 formed a more potent sulphoxide called timoprazole Ø It turned out that timoprazole prevented 10/22/2021 uptake of iodine by the thiroid Ø More analogues were prepared and picoprazole was found to be free from side effects 8
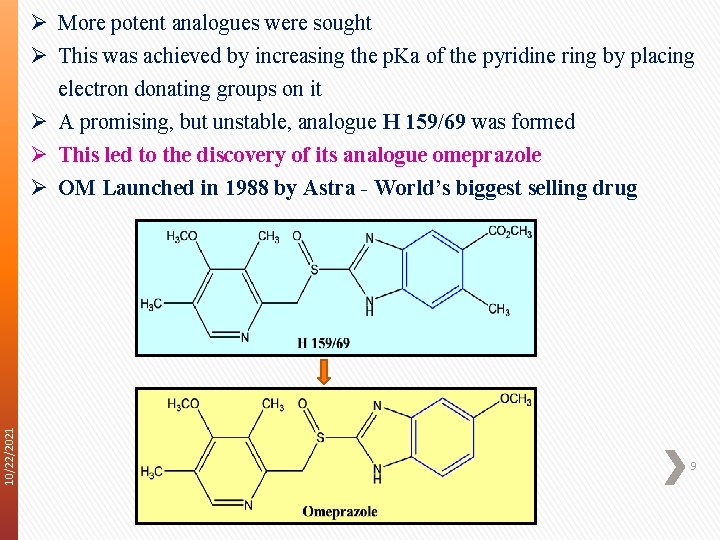
10/22/2021 Ø More potent analogues were sought Ø This was achieved by increasing the p. Ka of the pyridine ring by placing electron donating groups on it Ø A promising, but unstable, analogue H 159/69 was formed Ø This led to the discovery of its analogue omeprazole Ø OM Launched in 1988 by Astra - World’s biggest selling drug 9
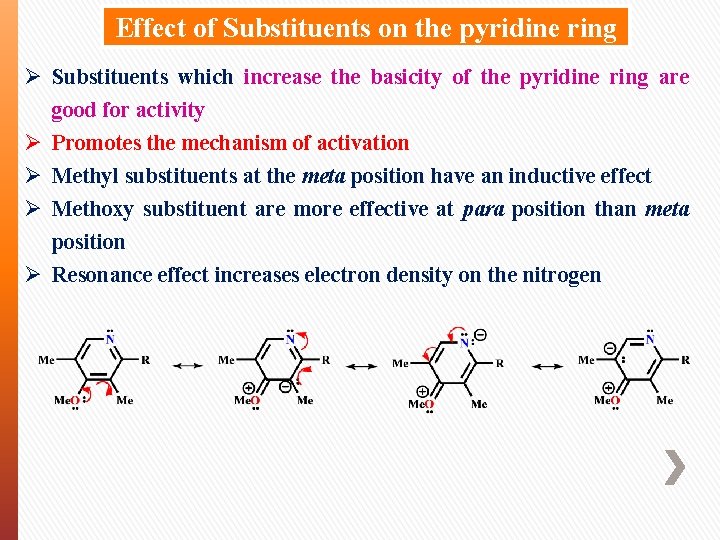
Effect of Substituents on the pyridine ring Ø Substituents which increase the basicity of the pyridine ring are good for activity Ø Promotes the mechanism of activation Ø Methyl substituents at the meta position have an inductive effect Ø Methoxy substituent are more effective at para position than meta position Ø Resonance effect increases electron density on the nitrogen
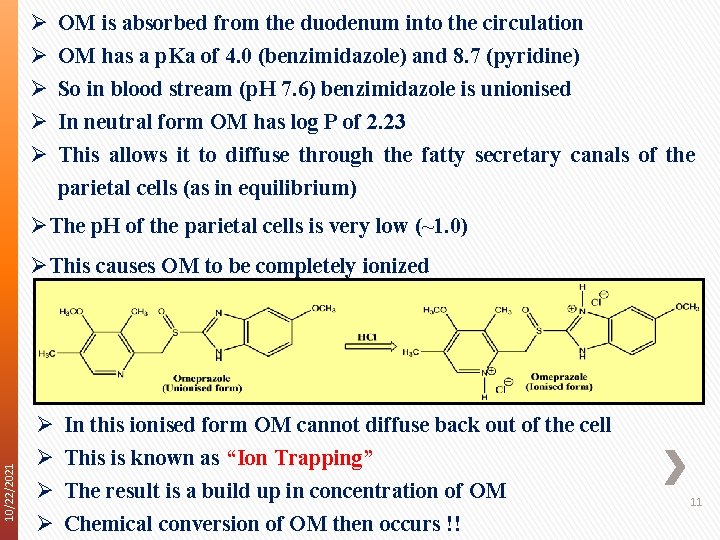
Ø Ø Ø OM is absorbed from the duodenum into the circulation OM has a p. Ka of 4. 0 (benzimidazole) and 8. 7 (pyridine) So in blood stream (p. H 7. 6) benzimidazole is unionised In neutral form OM has log P of 2. 23 This allows it to diffuse through the fatty secretary canals of the parietal cells (as in equilibrium) ØThe p. H of the parietal cells is very low (~1. 0) 10/22/2021 ØThis causes OM to be completely ionized Ø Ø In this ionised form OM cannot diffuse back out of the cell This is known as “Ion Trapping” The result is a build up in concentration of OM Chemical conversion of OM then occurs !! 11
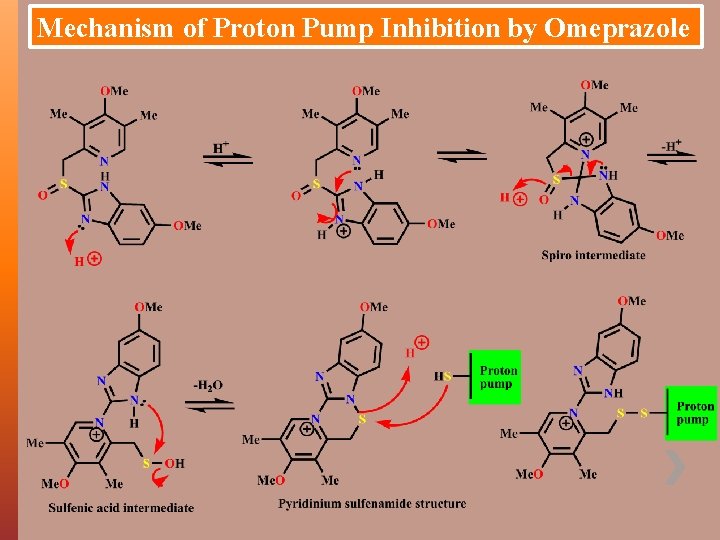
Mechanism of Proton Pump Inhibition by Omeprazole

10/22/2021 Mechanism of Proton Pump Inhibition by Omeprazole Ø Pyridine first ionised (p. Ka = 8. 7) by acid environment Ø Then in equilibrium the imidazole is protonated Ø Very rapidly the lone pair on pyridine N attacks edeficient 2 -carbon atom of the benzimidazole ring Ø This C sits between 2 N atoms one of which has a + charge which want to loose Ø Ring system wants to regain aromaticity because it is energetically favourable Ø It does this by forming the sulphenic acid Ø Lone pair on N attackes the neighbouring S atom Ø This causes the loss of water which gives the sulphenamide Ø There is maximal conversion to the sulphenamide due to the steep proton gradient caused by the H+/K+- ATPase enzyme 13 Ø There is ion trapping of both OM and the sulphenamide Ø This is why the drug acts specifically in the parietal cells
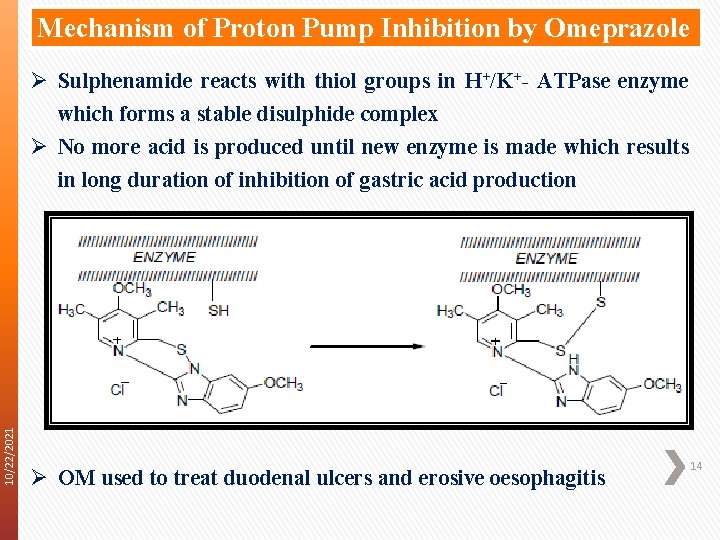
Mechanism of Proton Pump Inhibition by Omeprazole 10/22/2021 Ø Sulphenamide reacts with thiol groups in H+/K+- ATPase enzyme which forms a stable disulphide complex Ø No more acid is produced until new enzyme is made which results in long duration of inhibition of gastric acid production Ø OM used to treat duodenal ulcers and erosive oesophagitis 14
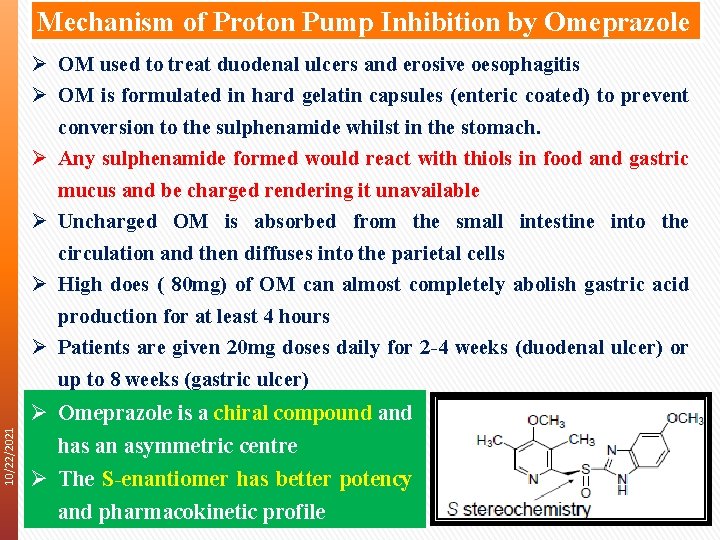
Mechanism of Proton Pump Inhibition by Omeprazole 10/22/2021 Ø OM used to treat duodenal ulcers and erosive oesophagitis Ø OM is formulated in hard gelatin capsules (enteric coated) to prevent conversion to the sulphenamide whilst in the stomach. Ø Any sulphenamide formed would react with thiols in food and gastric mucus and be charged rendering it unavailable Ø Uncharged OM is absorbed from the small intestine into the circulation and then diffuses into the parietal cells Ø High does ( 80 mg) of OM can almost completely abolish gastric acid production for at least 4 hours Ø Patients are given 20 mg doses daily for 2 -4 weeks (duodenal ulcer) or up to 8 weeks (gastric ulcer) Ø Omeprazole is a chiral compound and has an asymmetric centre Ø The S-enantiomer has better potency and pharmacokinetic profile 15
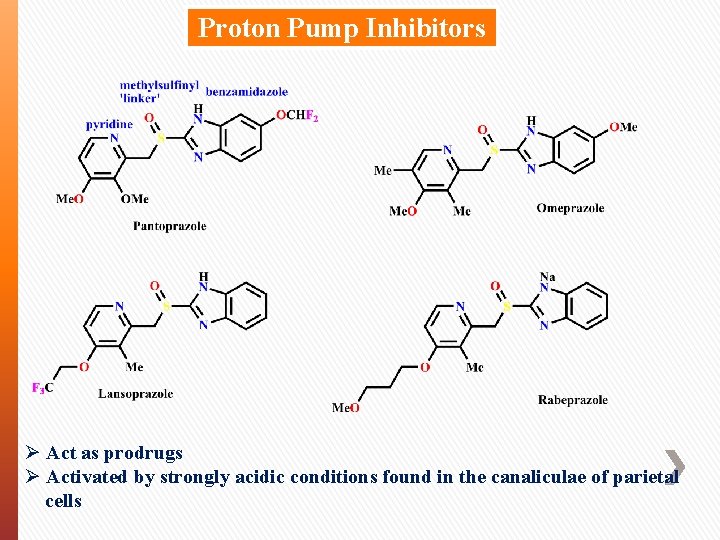
Proton Pump Inhibitors Ø Act as prodrugs Ø Activated by strongly acidic conditions found in the canaliculae of parietal cells

10/22/2021 Summary Ø OM localises in parietal cells where stomach acid is produced Ø It inhibits H+ /K+- ATPase enzyme which catalyses the final step of stomach acid production Ø In its unionised form it is absorbed into the blood from the duodenum Ø As it’s benzimidazole has a p. Ka = 4. 0 it remains in equilibrium in the blood (p. H = 7. 6) with a log. P = 2. 23 Ø Having a log. P = 2. 23 allows OM to diffuse though the fatty parietal cell where there is a p. H = 1. 0 Ø OM then ionises and becomes “trapped” and a build up in concentration of the drug occurs Ø Once in the ionised form a chemical conversion occurs which turns OM into the suphenamide (active form) Ø Sulphenamide reacts with H+/K+-ATPase enzyme and stops acid production Ø As no more acid can be produced until more enzyme is made OM has a long 17 lasting action Ø OM is a chiral molecule with the S-enantiomer being the active stereoisomer which was introduced to the clinic

10/22/2021 Thank you……. . 18
- Slides: 18