Protocol Deviations Initiative Overview For Member Company Use

Protocol Deviations Initiative Overview For Member Company Use Only Updated: August 2019

Trans. Celerate: A Not-for-Profit Entity Created to Foster Collaboration Our Shared Vision: To improve the health of people around the world by accelerating and simplifying the research and development of innovative new therapies. Copyright © 2019 Trans. Celerate Bio. Pharma Inc. , All rights reserved. 2
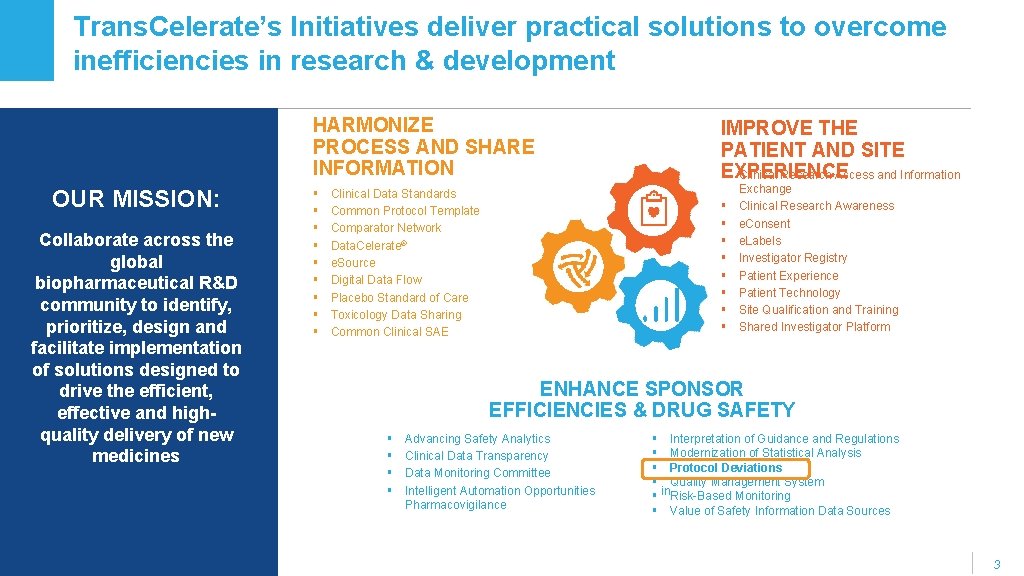
Trans. Celerate’s Initiatives deliver practical solutions to overcome inefficiencies in research & development HARMONIZE PROCESS AND SHARE INFORMATION OUR MISSION: Collaborate across the global biopharmaceutical R&D community to identify, prioritize, design and facilitate implementation of solutions designed to drive the efficient, effective and highquality delivery of new medicines Copyright © 2019 Trans. Celerate Bio. Pharma Inc. , All rights reserved. § § § § § Clinical Data Standards Common Protocol Template Comparator Network Data. Celerate® e. Source Digital Data Flow Placebo Standard of Care Toxicology Data Sharing Common Clinical SAE IMPROVE THE PATIENT AND SITE §EXPERIENCE Clinical Research Access and Information Site/Investigator Experience § § § § Exchange Clinical Research Awareness e. Consent e. Labels Investigator Registry Patient Experience Patient Technology Site Qualification and Training Shared Investigator Platform ENHANCE SPONSOR EFFICIENCIES & DRUG SAFETY § § Advancing Safety Analytics Clinical Data Transparency Data Monitoring Committee Intelligent Automation Opportunities Pharmacovigilance § Interpretation of Guidance and Regulations § Modernization of Statistical Analysis § Protocol Deviations § Quality Management System § in. Risk-Based Monitoring § Value of Safety Information Data Sources 3
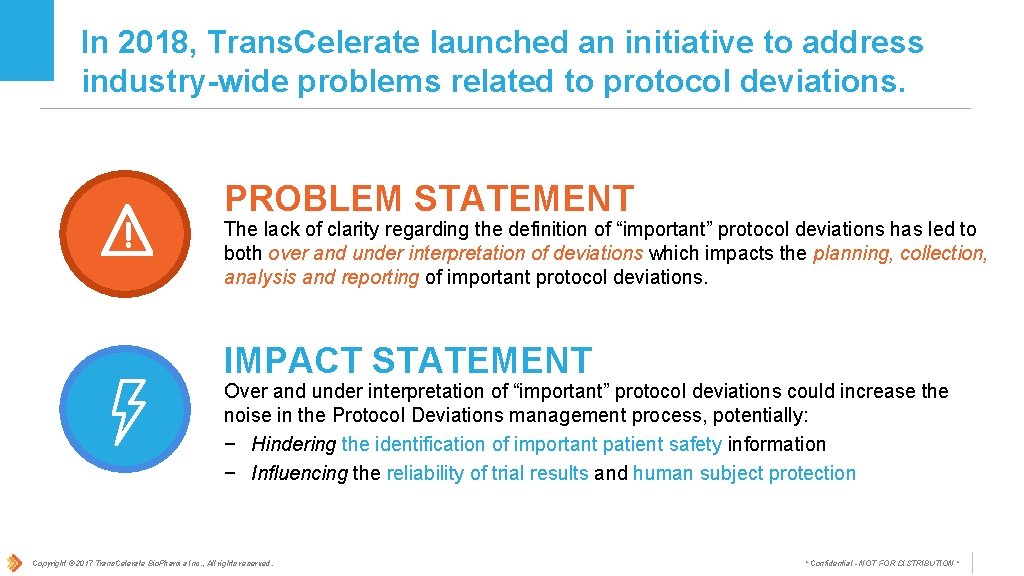
In 2018, Trans. Celerate launched an initiative to address industry-wide problems related to protocol deviations. PROBLEM STATEMENT The lack of clarity regarding the definition of “important” protocol deviations has led to both over and under interpretation of deviations which impacts the planning, collection, analysis and reporting of important protocol deviations. IMPACT STATEMENT Over and under interpretation of “important” protocol deviations could increase the noise in the Protocol Deviations management process, potentially: − Hindering the identification of important patient safety information − Influencing the reliability of trial results and human subject protection Copyright © 2017 Trans. Celerate Bio. Pharma Inc. , All rights reserved. * Confidential - NOT FOR DISTRIBUTION *
Surveys of Trans. Celerate Membership confirmed wide variation in terminology and challenges in multiple areas Copyright © 2017 Trans. Celerate Bio. Pharma Inc. , All rights reserved. * Confidential - NOT FOR DISTRIBUTION *

Survey of Stakeholders confirmed challenges with current state of Protocol Deviations management. Site Landscape Survey Findings q Variations in Protocol Deviations definition between sponsors can lead to increased burden for sites, reducing time investigators can devote to patients. q Sites reported variations in definition and/or process can increase the complexity of, and cause delays and inaccuracies in, protocol deviation reporting to sponsors. IRB/IEC Survey Findings q Confirmed observations from sites and sponsors Summary Protocol Deviation (PD) Management processes are complex, varied and broadly impacted by a wide range of definitions for identification and classification. These differences are associated with process inefficiencies across both sites and sponsors which can hinder rapid identification of important Protocol Deviations. Copyright © 2017 Trans. Celerate Bio. Pharma Inc. , All rights reserved. * Confidential - NOT FOR DISTRIBUTION *

Objectives of initiative Reduce noise to support rapid identification of important protocol deviations Increase process efficiencies at site and sponsor BY Improving interpretation of guidelines, including definition Improving management of protocol deviation processes Engaging with the FDA and other Health Authorities on these critical issues and solutions. Copyright © 2017 Trans. Celerate Bio. Pharma Inc. , All rights reserved. * Confidential - NOT FOR DISTRIBUTION *
Key principles considered Build on the ICH E 3 Q&A R 1 of definition “Protocol Deviation” The event occurred (i. e. , not theoretical) The event is related to data point or process identified in the protocol or documents referenced in the protocol Protocol deviations are independent of fault, blame or circumstance Incorporate ICH E 6 R 2 into proposed definition of “important protocol deviations” Important protocol deviations as a subset of protocol deviations and which may significantly impact “key or critical” study data Copyright © 2017 Trans. Celerate Bio. Pharma Inc. , All rights reserved. * Confidential - NOT FOR DISTRIBUTION *
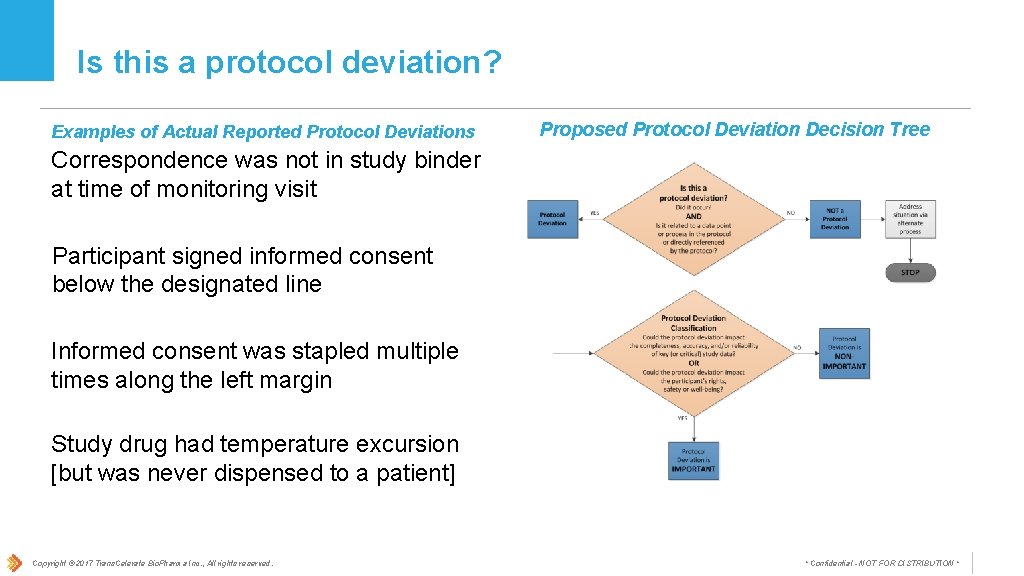
Is this a protocol deviation? Examples of Actual Reported Protocol Deviations Proposed Protocol Deviation Decision Tree Correspondence was not in study binder at time of monitoring visit Participant signed informed consent below the designated line Informed consent was stapled multiple times along the left margin Study drug had temperature excursion [but was never dispensed to a patient] Copyright © 2017 Trans. Celerate Bio. Pharma Inc. , All rights reserved. * Confidential - NOT FOR DISTRIBUTION *
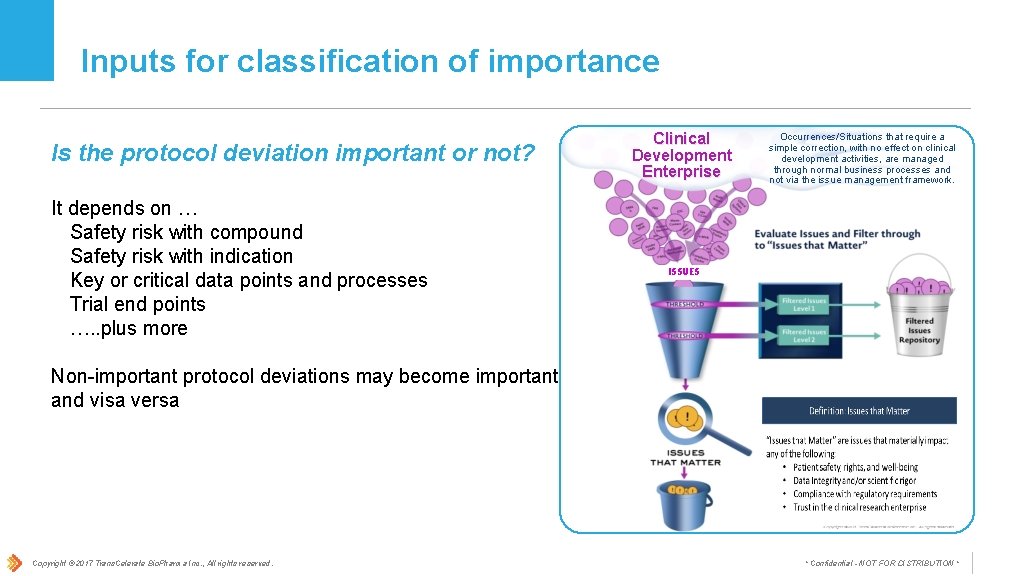
Inputs for classification of importance Is the protocol deviation important or not? It depends on … Safety risk with compound Safety risk with indication Key or critical data points and processes Trial end points …. . plus more Clinical Development Enterprise Occurrences/Situations that require a simple correction, with no effect on clinical development activities, are managed through normal business processes and not via the issue management framework. ISSUES Non-important protocol deviations may become important and visa versa Copyright © 2017 Trans. Celerate Bio. Pharma Inc. , All rights reserved. * Confidential - NOT FOR DISTRIBUTION *
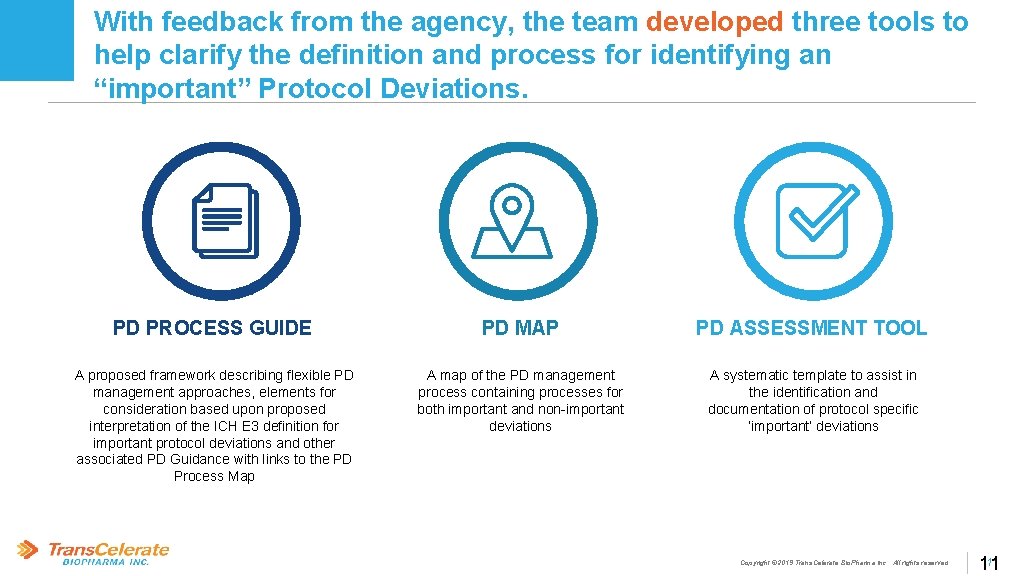
With feedback from the agency, the team developed three tools to help clarify the definition and process for identifying an “important” Protocol Deviations. PD PROCESS GUIDE PD MAP PD ASSESSMENT TOOL A proposed framework describing flexible PD management approaches, elements for consideration based upon proposed interpretation of the ICH E 3 definition for important protocol deviations and other associated PD Guidance with links to the PD Process Map A map of the PD management process containing processes for both important and non-important deviations A systematic template to assist in the identification and documentation of protocol specific ‘important’ deviations Copyright © 2019 Trans. Celerate Bio. Pharma Inc. , All rights reserved. 11 11
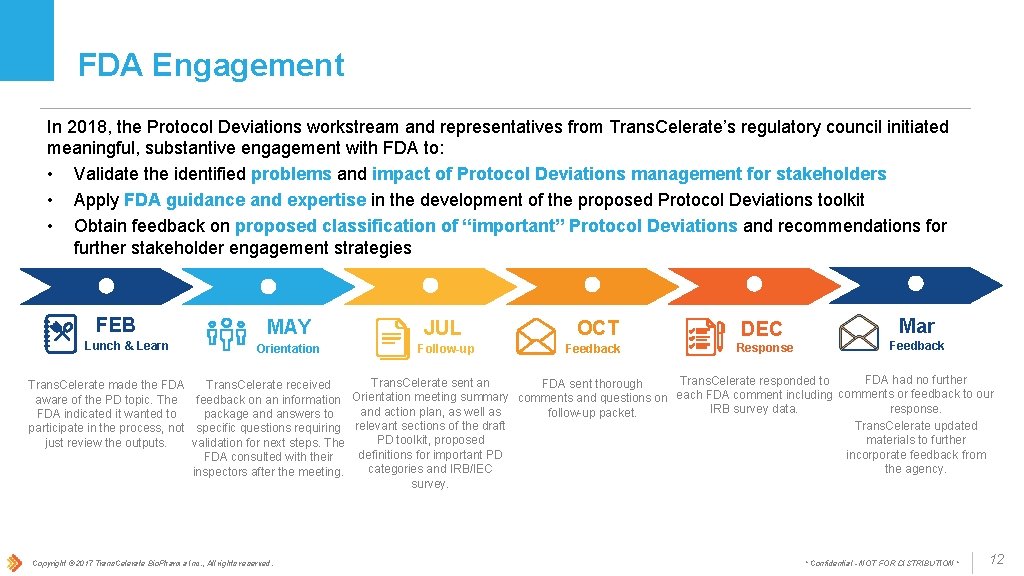
FDA Engagement In 2018, the Protocol Deviations workstream and representatives from Trans. Celerate’s regulatory council initiated meaningful, substantive engagement with FDA to: • Validate the identified problems and impact of Protocol Deviations management for stakeholders • Apply FDA guidance and expertise in the development of the proposed Protocol Deviations toolkit • Obtain feedback on proposed classification of “important” Protocol Deviations and recommendations for further stakeholder engagement strategies FEB Lunch & Learn MAY Orientation JUL Follow-up OCT Feedback DEC Response Mar Feedback FDA had no further Trans. Celerate responded to Trans. Celerate sent an FDA sent thorough Trans. Celerate made the FDA Trans. Celerate received aware of the PD topic. The feedback on an information Orientation meeting summary comments and questions on each FDA comment including comments or feedback to our response. IRB survey data. and action plan, as well as follow-up packet. FDA indicated it wanted to package and answers to Trans. Celerate updated participate in the process, not specific questions requiring relevant sections of the draft materials to further PD toolkit, proposed just review the outputs. validation for next steps. The incorporate feedback from definitions for important PD FDA consulted with their the agency. categories and IRB/IEC inspectors after the meeting. survey. Copyright © 2017 Trans. Celerate Bio. Pharma Inc. , All rights reserved. * Confidential - NOT FOR DISTRIBUTION * 12
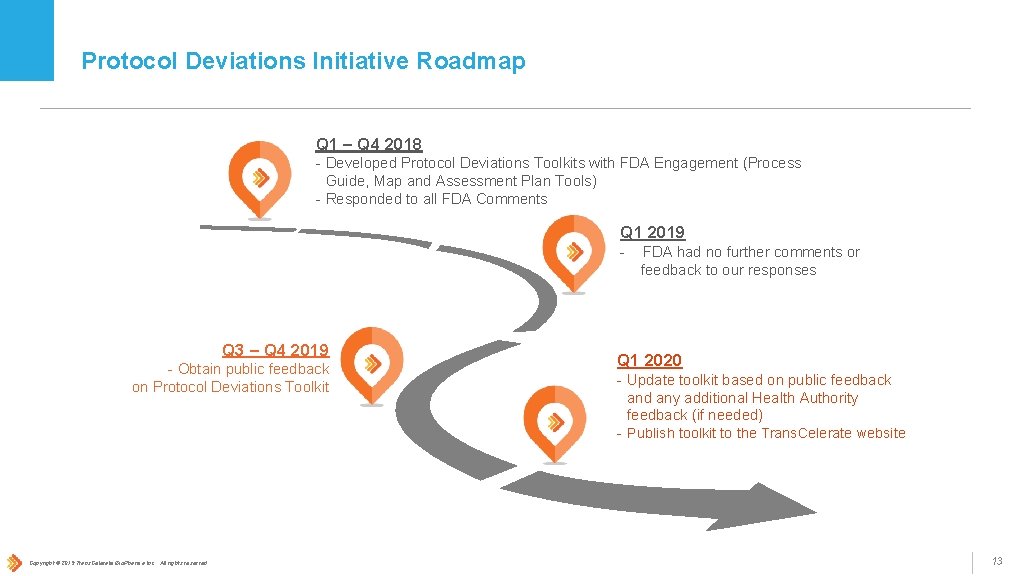
Protocol Deviations Initiative Roadmap Q 1 – Q 4 2018 - Developed Protocol Deviations Toolkits with FDA Engagement (Process Guide, Map and Assessment Plan Tools) - Responded to all FDA Comments Q 1 2019 - Q 3 – Q 4 2019 - Obtain public feedback on Protocol Deviations Toolkit Copyright © 2019 Trans. Celerate Bio. Pharma Inc. , All rights reserved. FDA had no further comments or feedback to our responses Q 1 2020 - Update toolkit based on public feedback and any additional Health Authority feedback (if needed) - Publish toolkit to the Trans. Celerate website 13

For more information about Trans. Celerate, visit us: www. Trans. Celerate. Bio. Pharma. Inc. com Watch our “About Us” Video Sign up for our Newsletter, Accelerate to Innovate @Trans. Celerate Bio. Pharma Inc.
- Slides: 14