Protocol Development Planning Your Review The Review Protocol











- Slides: 13

Protocol Development

Planning Your Review • The Review Protocol – What is the role of the review protocol? – Why is a review protocol necessary? – What information does it contain?

Role of the Protocol • The protocol is the first milestone of any systematic review • A (rigid) plan or framework for the review • A protocol helps to avoid or minimise bias – Bias may occur in the retrieval, selection, and appraisal of studies, as well as during the extraction, evaluation and interpretation of data • A protocol can (and should be) sent for external peer review

Protocol Structure and Content • Overview – Background – Review questions/ objectives • Methods – – – Search strategy Selection criteria Quality assessment Data extraction Data synthesis • Conduct issues – Peer review process – Project timetable – Dissemination strategy – Individual responsibilities

Background section • Sets the context and provides the rationale for the review: – Patients / disease characteristics, e. g. epidemiology – Importance: clinical and public health implications – Current practice – Alternative interventions – Available evidence, including existing systematic reviews

Research questions/ objectives • Precise formulation in relation to key components (PICO): – Population/ patients / problem – Intervention, or exposure – Comparison group – Outcome(s) of interest

Search Strategy • The protocol should make it clear how relevant studies are going to be identified: – Specify which databases (including dates) and other sources will be searched – Identify key search terms

Study Selection • The protocol should make it clear how studies will be selected for inclusion in the review: – Specify the inclusion (and exclusion) criteria – Report details of selection process • How many reviewers involved? • Will the process be completed independently? • How will disagreements be resolved?

Quality Assessment • The protocol should make it clear why and how included studies will be quality assessed: – State the purpose of quality assessment, e. g. is it for selection, data synthesis, interpretation of results? – Describe the criteria / checklist to be used – Detail the quality assessment process / procedure • How many reviewers involved? • Will the process be completed independently? • How will disagreements be resolved?

Data Extraction • The protocol should report: – What data are to be extracted from primary studies – A draft template for data extraction – How data will be presented in the report – Any planned manipulations of study data, e. g. calculation of summary statistics, ITT-analysis, etc. – The process of data extraction • How many reviewers will be involved? • How will disagreements be resolved?

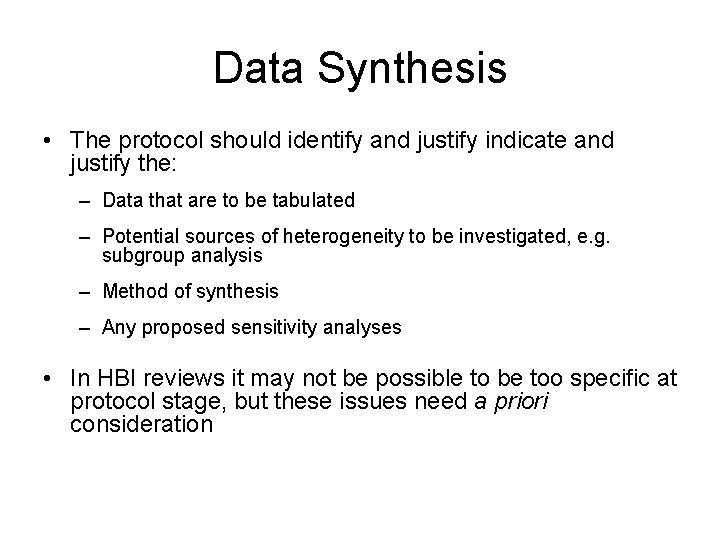
Data Synthesis • The protocol should identify and justify indicate and justify the: – Data that are to be tabulated – Potential sources of heterogeneity to be investigated, e. g. subgroup analysis – Method of synthesis – Any proposed sensitivity analyses • In HBI reviews it may not be possible to be too specific at protocol stage, but these issues need a priori consideration

Dissemination • Failing to disseminate research findings is unethical, and the protocol should detail precisely a dissemination strategy: – How will you disseminate the findings? – Where will you publish? – How will you publish your review? – In what format will you publish? – Who is your intended audience?

Peer review / Advisory Group • Reviewers should establish an Advisory Group who can provide peer-review at different stages of the review process – Topic experts (including ‘users’) will be able to comment on the relevance of the review question, as well as possible sources of heterogeneity – Methodological experts will be able to comment on whether the proposed methods will answer the review questions • Trainees must obtain protocol approval before starting their SR