Proteomic Analysis of Ocular Discharges and Plasma From
- Slides: 1

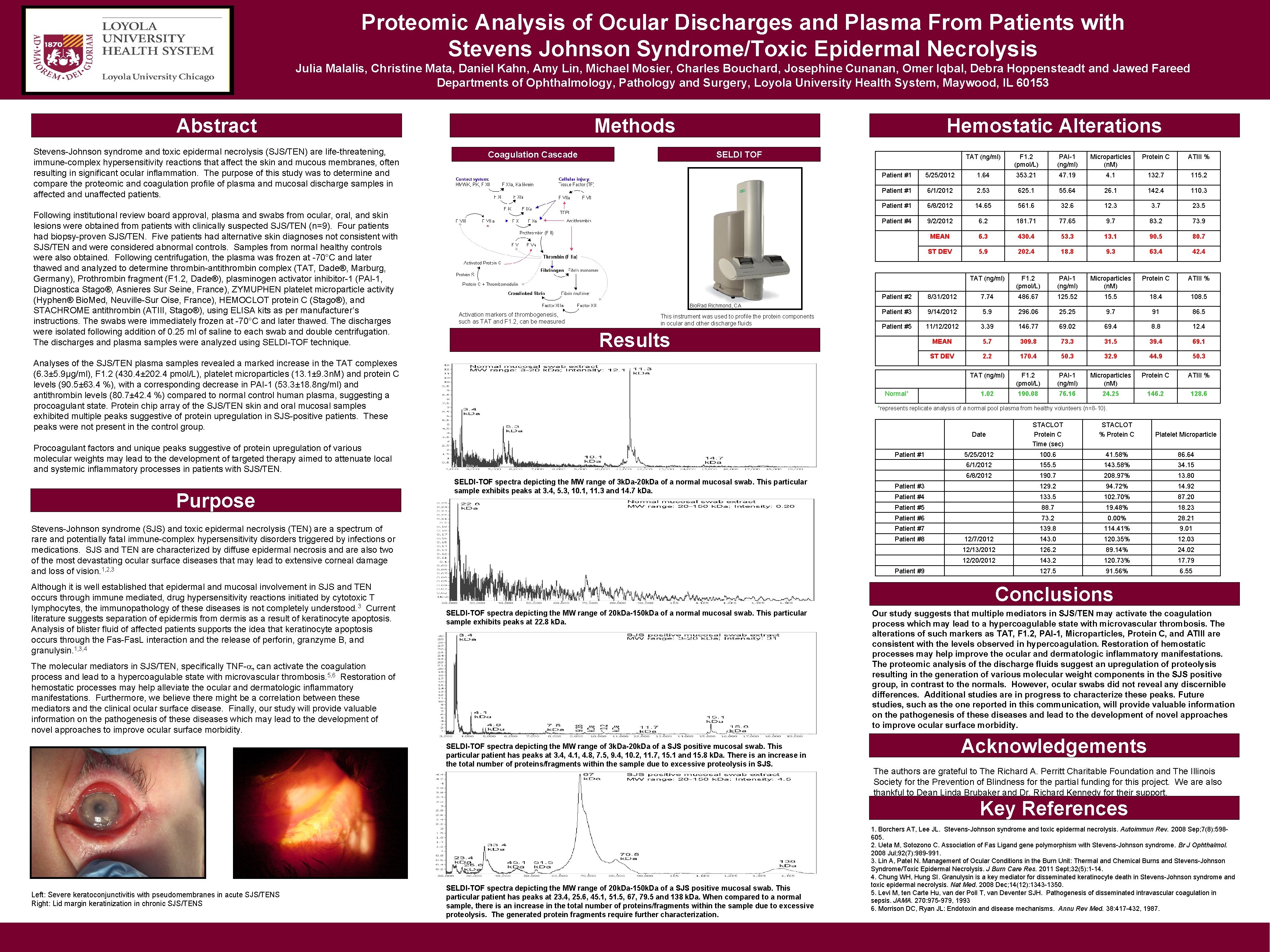
Proteomic Analysis of Ocular Discharges and Plasma From Patients with Stevens Johnson Syndrome/Toxic Epidermal Necrolysis Julia Malalis, Christine Mata, Daniel Kahn, Amy Lin, Michael Mosier, Charles Bouchard, Josephine Cunanan, Omer Iqbal, Debra Hoppensteadt and Jawed Fareed Departments of Ophthalmology, Pathology and Surgery, Loyola University Health System, Maywood, IL 60153 Abstract Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN) are life-threatening, immune-complex hypersensitivity reactions that affect the skin and mucous membranes, often resulting in significant ocular inflammation. The purpose of this study was to determine and compare the proteomic and coagulation profile of plasma and mucosal discharge samples in affected and unaffected patients. Following institutional review board approval, plasma and swabs from ocular, oral, and skin lesions were obtained from patients with clinically suspected SJS/TEN (n=9). Four patients had biopsy-proven SJS/TEN. Five patients had alternative skin diagnoses not consistent with SJS/TEN and were considered abnormal controls. Samples from normal healthy controls were also obtained. Following centrifugation, the plasma was frozen at -70°C and later thawed analyzed to determine thrombin-antithrombin complex (TAT, Dade®, Marburg, Germany), Prothrombin fragment (F 1. 2, Dade®), plasminogen activator inhibitor-1 (PAI-1, Diagnostica Stago®, Asnieres Sur Seine, France), ZYMUPHEN platelet microparticle activity (Hyphen® Bio. Med, Neuville-Sur Oise, France), HEMOCLOT protein C (Stago®), and STACHROME antithrombin (ATIII, Stago®), using ELISA kits as per manufacturer’s instructions. The swabs were immediately frozen at -70°C and later thawed. The discharges were isolated following addition of 0. 25 ml of saline to each swab and double centrifugation. The discharges and plasma samples were analyzed using SELDI-TOF technique. Methods Coagulation Cascade Hemostatic Alterations SELDI TOF Bio. Rad Richmond, CA Activation markers of thrombogenesis, such as TAT and F 1. 2, can be measured This instrument was used to profile the protein components in ocular and other discharge fluids Results Analyses of the SJS/TEN plasma samples revealed a marked increase in the TAT complexes (6. 3± 5. 9µg/ml), F 1. 2 (430. 4± 202. 4 pmol/L), platelet microparticles (13. 1± 9. 3 n. M) and protein C levels (90. 5± 63. 4 %), with a corresponding decrease in PAI-1 (53. 3± 18. 8 ng/ml) and antithrombin levels (80. 7± 42. 4 %) compared to normal control human plasma, suggesting a procoagulant state. Protein chip array of the SJS/TEN skin and oral mucosal samples exhibited multiple peaks suggestive of protein upregulation in SJS-positive patients. These peaks were not present in the control group. PAI-1 (ng/ml) Microparticles (n. M) Protein C ATIII % 5/25/2012 1. 64 353. 21 47. 19 4. 1 132. 7 115. 2 Patient #1 6/1/2012 2. 53 625. 1 55. 64 26. 1 142. 4 110. 3 Patient #1 6/8/2012 14. 65 561. 6 32. 6 12. 3 3. 7 23. 5 Patient #4 9/2/2012 6. 2 181. 71 77. 65 9. 7 83. 2 73. 9 MEAN 6. 3 430. 4 53. 3 13. 1 90. 5 80. 7 ST DEV 5. 9 202. 4 18. 8 9. 3 63. 4 42. 4 TAT (ng/ml) F 1. 2 (pmol/L) PAI-1 (ng/ml) Microparticles (n. M) Protein C ATIII % Patient #2 8/31/2012 7. 74 486. 67 125. 52 15. 5 18. 4 108. 5 Patient #3 9/14/2012 5. 9 296. 06 25. 25 9. 7 91 86. 5 Patient #5 11/12/2012 3. 39 146. 77 69. 02 69. 4 8. 8 12. 4 MEAN 5. 7 309. 8 73. 3 31. 5 39. 4 69. 1 ST DEV 2. 2 170. 4 50. 3 32. 9 44. 9 50. 3 TAT (ng/ml) F 1. 2 (pmol/L) PAI-1 (ng/ml) Microparticles (n. M) Protein C ATIII % 1. 02 190. 08 76. 16 24. 25 146. 2 128. 6 *represents replicate analysis of a normal pool plasma from healthy volunteers (n=8 -10). Date STACLOT Protein C Time (sec) STACLOT % Protein C Platelet Microparticle 5/25/2012 100. 6 41. 58% 86. 64 6/1/2012 155. 5 143. 58% 34. 15 6/8/2012 190. 7 208. 97% 13. 80 Patient #3 129. 2 94. 72% 14. 92 Patient #4 133. 5 102. 70% 87. 20 Patient #5 88. 7 19. 48% 18. 23 Patient #6 73. 2 0. 00% 28. 21 Patient #7 139. 8 114. 41% 9. 01 12/7/2012 143. 0 120. 35% 12. 03 12/13/2012 126. 2 89. 14% 24. 02 12/20/2012 143. 2 120. 73% 17. 79 127. 5 91. 56% 6. 55 Patient #1 SELDI-TOF spectra depicting the MW range of 3 k. Da-20 k. Da of a normal mucosal swab. This particular sample exhibits peaks at 3. 4, 5. 3, 10. 1, 11. 3 and 14. 7 k. Da. Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are a spectrum of rare and potentially fatal immune-complex hypersensitivity disorders triggered by infections or medications. SJS and TEN are characterized by diffuse epidermal necrosis and are also two of the most devastating ocular surface diseases that may lead to extensive corneal damage and loss of vision. 1, 2, 3 Although it is well established that epidermal and mucosal involvement in SJS and TEN occurs through immune mediated, drug hypersensitivity reactions initiated by cytotoxic T lymphocytes, the immunopathology of these diseases is not completely understood. 3 Current literature suggests separation of epidermis from dermis as a result of keratinocyte apoptosis. Analysis of blister fluid of affected patients supports the idea that keratinocyte apoptosis occurs through the Fas-Fas. L interaction and the release of perforin, granzyme B, and granulysin. 1, 3, 4 F 1. 2 (pmol/L) Patient #1 Normal* Procoagulant factors and unique peaks suggestive of protein upregulation of various molecular weights may lead to the development of targeted therapy aimed to attenuate local and systemic inflammatory processes in patients with SJS/TEN. Purpose TAT (ng/ml) Patient #8 Patient #9 Conclusions SELDI-TOF spectra depicting the MW range of 20 k. Da-150 k. Da of a normal mucosal swab. This particular sample exhibits peaks at 22. 8 k. Da. The molecular mediators in SJS/TEN, specifically TNF-a, can activate the coagulation process and lead to a hypercoagulable state with microvascular thrombosis. 5, 6 Restoration of hemostatic processes may help alleviate the ocular and dermatologic inflammatory manifestations. Furthermore, we believe there might be a correlation between these mediators and the clinical ocular surface disease. Finally, our study will provide valuable information on the pathogenesis of these diseases which may lead to the development of novel approaches to improve ocular surface morbidity. SELDI-TOF spectra depicting the MW range of 3 k. Da-20 k. Da of a SJS positive mucosal swab. This particular patient has peaks at 3. 4, 4. 1, 4. 8, 7. 5, 9. 4, 10. 2, 11. 7, 15. 1 and 15. 8 k. Da. There is an increase in the total number of proteins/fragments within the sample due to excessive proteolysis in SJS. Our study suggests that multiple mediators in SJS/TEN may activate the coagulation process which may lead to a hypercoagulable state with microvascular thrombosis. The alterations of such markers as TAT, F 1. 2, PAI-1, Microparticles, Protein C, and ATIII are consistent with the levels observed in hypercoagulation. Restoration of hemostatic processes may help improve the ocular and dermatologic inflammatory manifestations. The proteomic analysis of the discharge fluids suggest an upregulation of proteolysis resulting in the generation of various molecular weight components in the SJS positive group, in contrast to the normals. However, ocular swabs did not reveal any discernible differences. Additional studies are in progress to characterize these peaks. Future studies, such as the one reported in this communication, will provide valuable information on the pathogenesis of these diseases and lead to the development of novel approaches to improve ocular surface morbidity. Acknowledgements The authors are grateful to The Richard A. Perritt Charitable Foundation and The Illinois Society for the Prevention of Blindness for the partial funding for this project. We are also thankful to Dean Linda Brubaker and Dr. Richard Kennedy for their support. Key References Left: Severe keratoconjunctivitis with pseudomembranes in acute SJS/TENS Right: Lid margin keratinization in chronic SJS/TENS SELDI-TOF spectra depicting the MW range of 20 k. Da-150 k. Da of a SJS positive mucosal swab. This particular patient has peaks at 23. 4, 25. 6, 45. 1, 51. 5, 67, 79. 5 and 138 k. Da. When compared to a normal sample, there is an increase in the total number of proteins/fragments within the sample due to excessive proteolysis. The generated protein fragments require further characterization. 1. Borchers AT, Lee JL. Stevens-Johnson syndrome and toxic epidermal necrolysis. Autoimmun Rev. 2008 Sep; 7(8): 598605. 2. Ueta M, Sotozono C. Association of Fas Ligand gene polymorphism with Stevens-Johnson syndrome. Br J Ophthalmol. 2008 Jul; 92(7): 989 -991. 3. Lin A, Patel N. Management of Ocular Conditions in the Burn Unit: Thermal and Chemical Burns and Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. J Burn Care Res. 2011 Sept; 32(5): 1 -14. 4. Chung WH, Hung SI. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008 Dec; 14(12): 1343 -1350. 5. Levi M, ten Carte Hu, van der Poll T, van Deventer SJH. Pathogenesis of disseminated intravascular coagulation in sepsis. JAMA. 270: 975 -979, 1993 6. Morrison DC, Ryan JL: Endotoxin and disease mechanisms. Annu Rev Med. 38: 417 -432, 1987.

