Proteins Structure Elements Carbon C Hydrogen H Oxygen
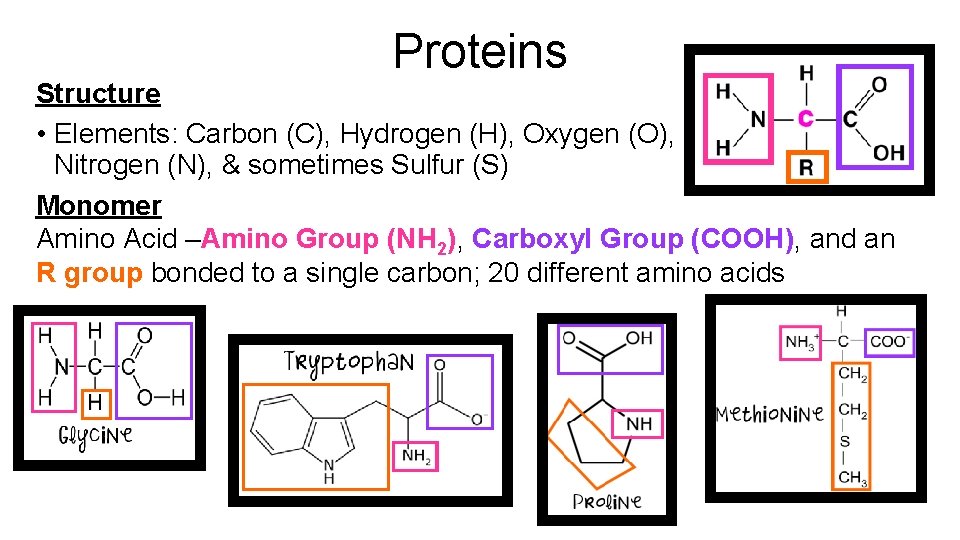
Proteins Structure • Elements: Carbon (C), Hydrogen (H), Oxygen (O), Nitrogen (N), & sometimes Sulfur (S) Monomer Amino Acid –Amino Group (NH 2), Carboxyl Group (COOH), and an R group bonded to a single carbon; 20 different amino acids
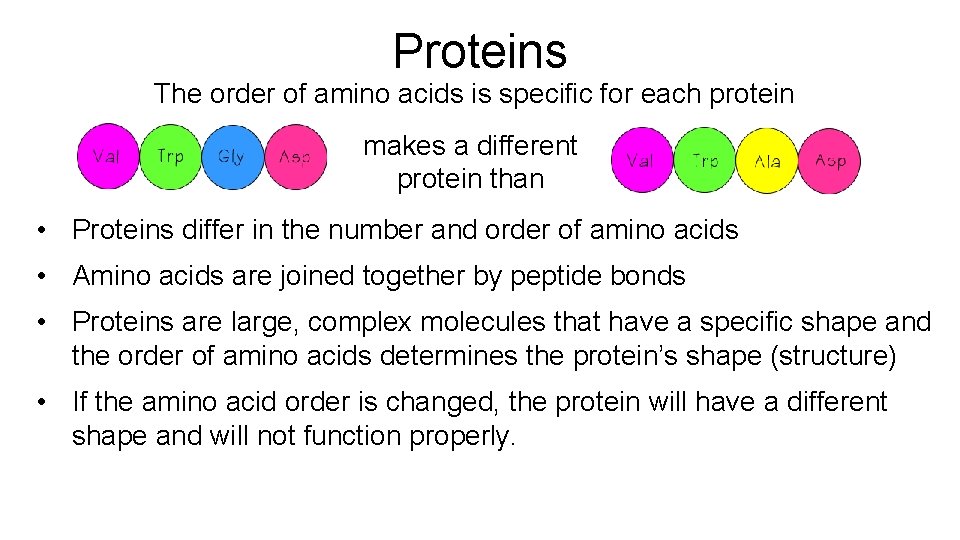
Proteins The order of amino acids is specific for each protein makes a different protein than • Proteins differ in the number and order of amino acids • Amino acids are joined together by peptide bonds • Proteins are large, complex molecules that have a specific shape and the order of amino acids determines the protein’s shape (structure) • If the amino acid order is changed, the protein will have a different shape and will not function properly.
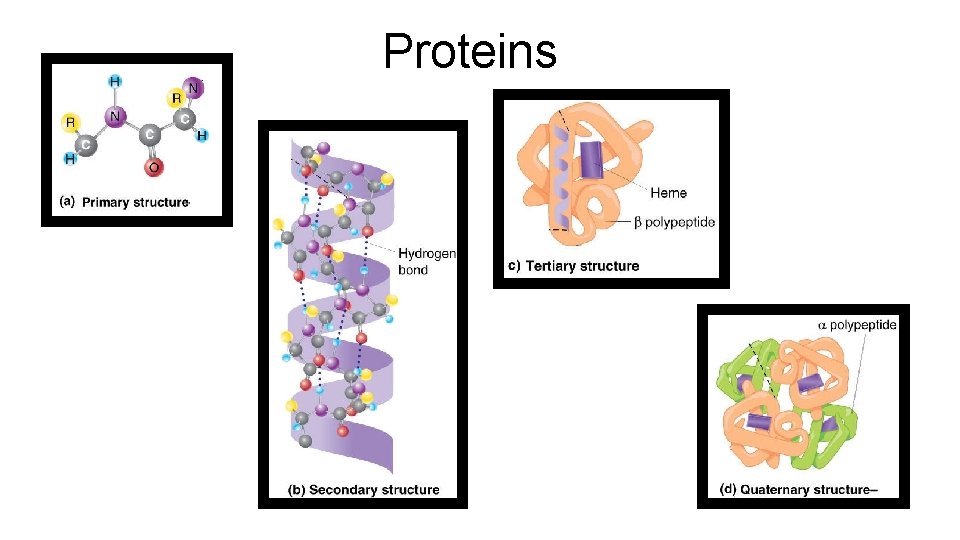
Proteins
Proteins Function • Proteins are a critical component of every single cell in your body & have a wide range of functions • SOME functions: growth, support (connective tissues), movement (muscles), transport of molecules in & out of cells, transport of molecules around body, enzymes, fight infections, energy*, hormones * Your body will not use proteins for energy unless you are not eating enough carbs & lipids for your energy requirements. Proteins are critically important and your body will not break them down for energy unless it has no other option.

Proteins Examples
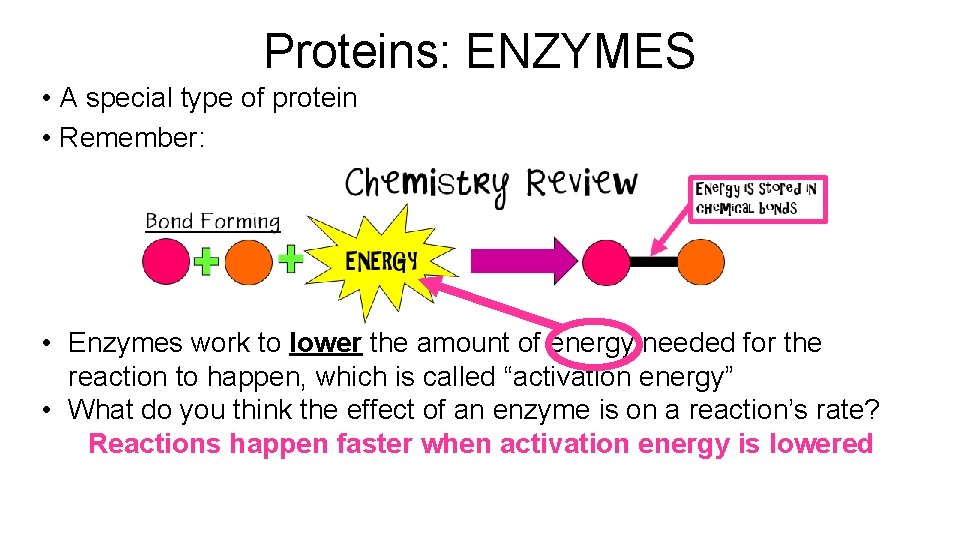
Proteins: ENZYMES • A special type of protein • Remember: • Enzymes work to lower the amount of energy needed for the reaction to happen, which is called “activation energy” • What do you think the effect of an enzyme is on a reaction’s rate? Reactions happen faster when activation energy is lowered
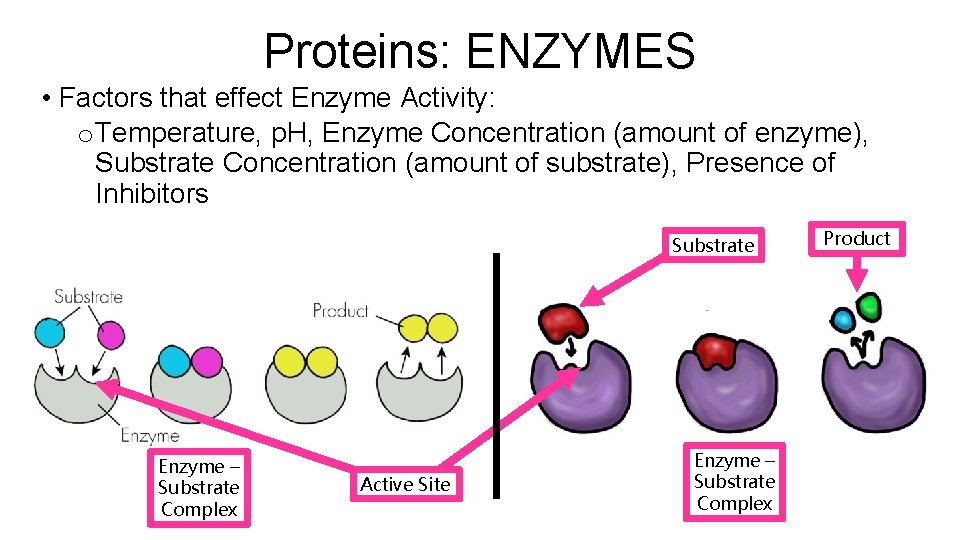
Proteins: ENZYMES • Factors that effect Enzyme Activity: o Temperature, p. H, Enzyme Concentration (amount of enzyme), Substrate Concentration (amount of substrate), Presence of Inhibitors Substrate Enzyme – Substrate Complex Active Site Enzyme – Substrate Complex Product
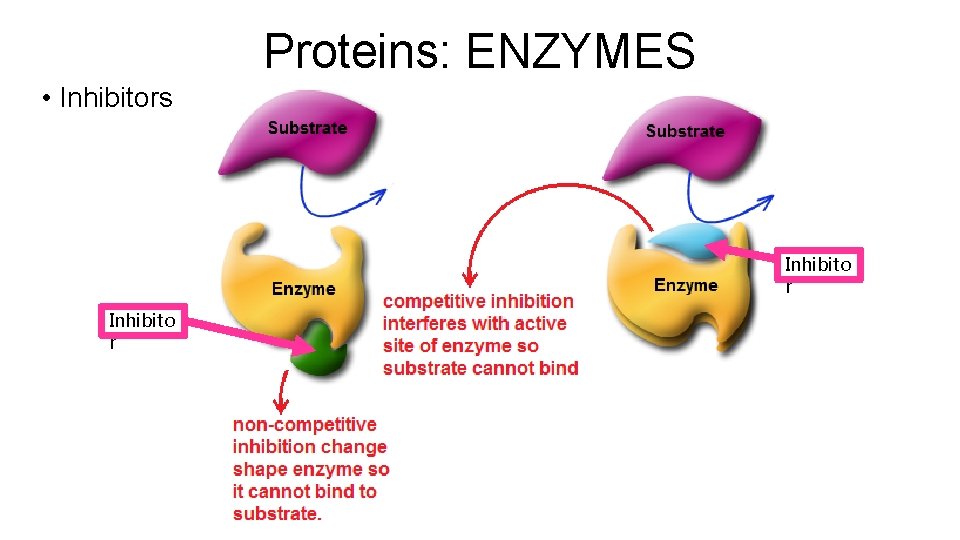
Proteins: ENZYMES • Inhibitors Inhibito r
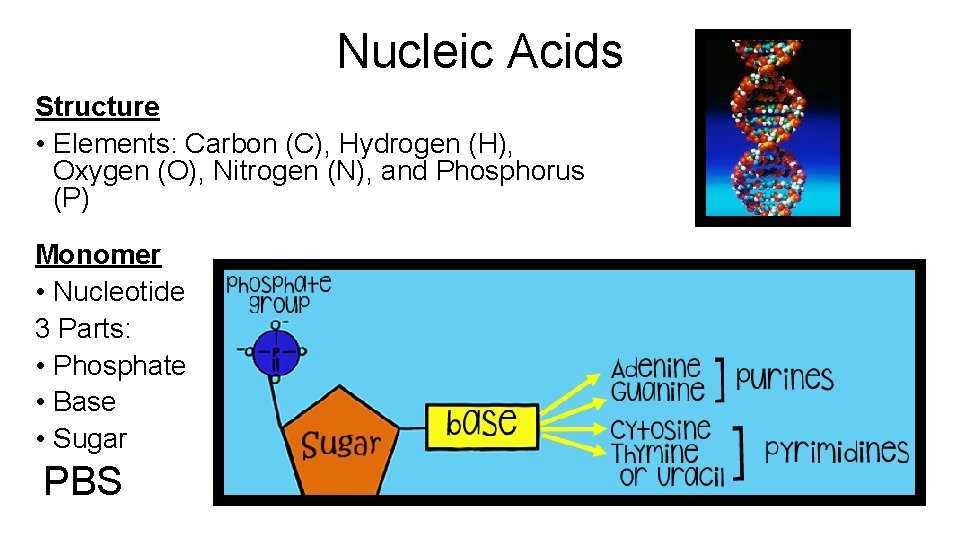
Nucleic Acids Structure • Elements: Carbon (C), Hydrogen (H), Oxygen (O), Nitrogen (N), and Phosphorus (P) Monomer • Nucleotide 3 Parts: • Phosphate • Base • Sugar PBS
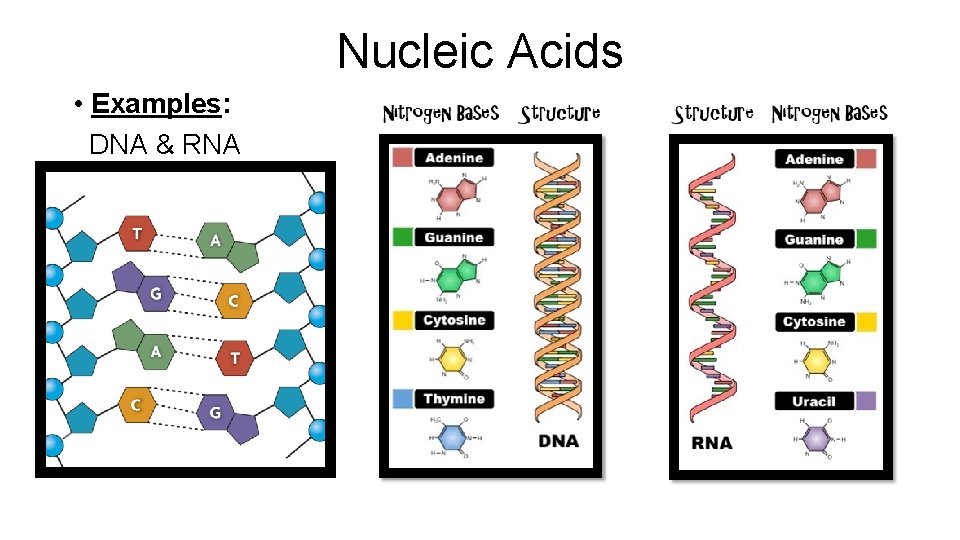
Nucleic Acids • Examples: DNA & RNA
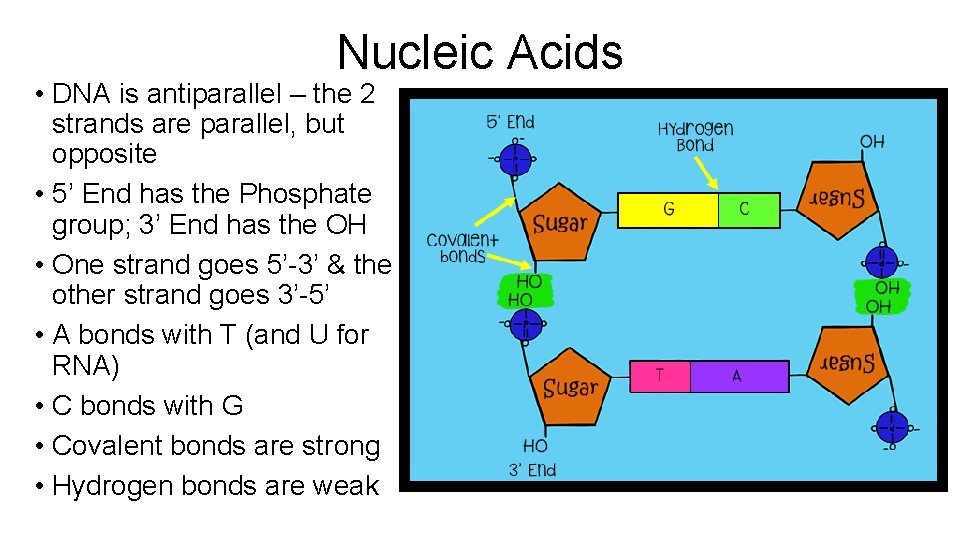
Nucleic Acids • DNA is antiparallel – the 2 strands are parallel, but opposite • 5’ End has the Phosphate group; 3’ End has the OH • One strand goes 5’-3’ & the other strand goes 3’-5’ • A bonds with T (and U for RNA) • C bonds with G • Covalent bonds are strong • Hydrogen bonds are weak
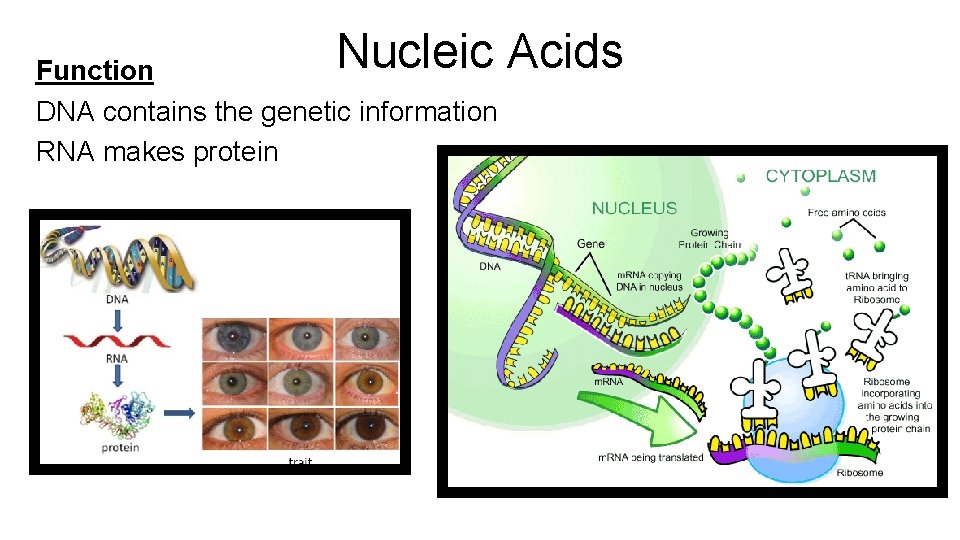
Nucleic Acids Function DNA contains the genetic information RNA makes protein
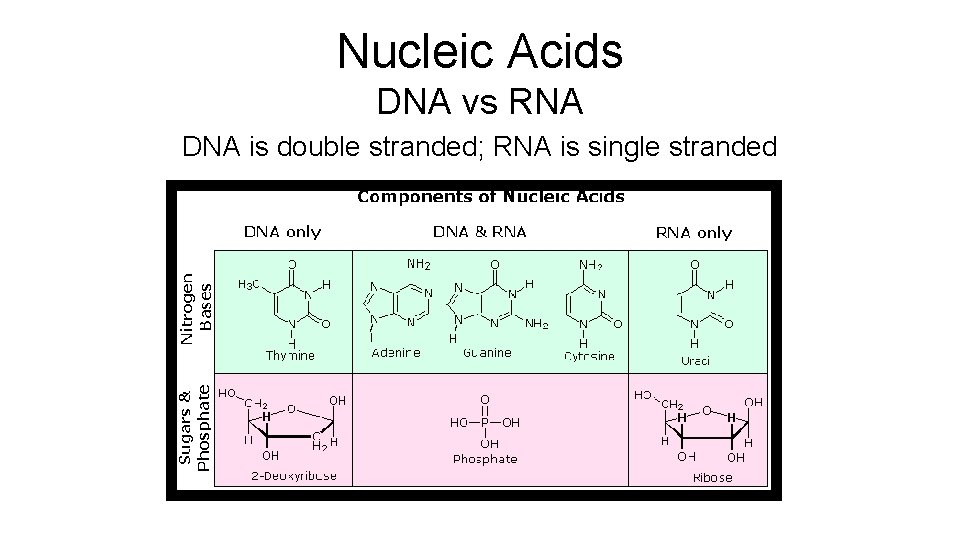
Nucleic Acids DNA vs RNA DNA is double stranded; RNA is single stranded
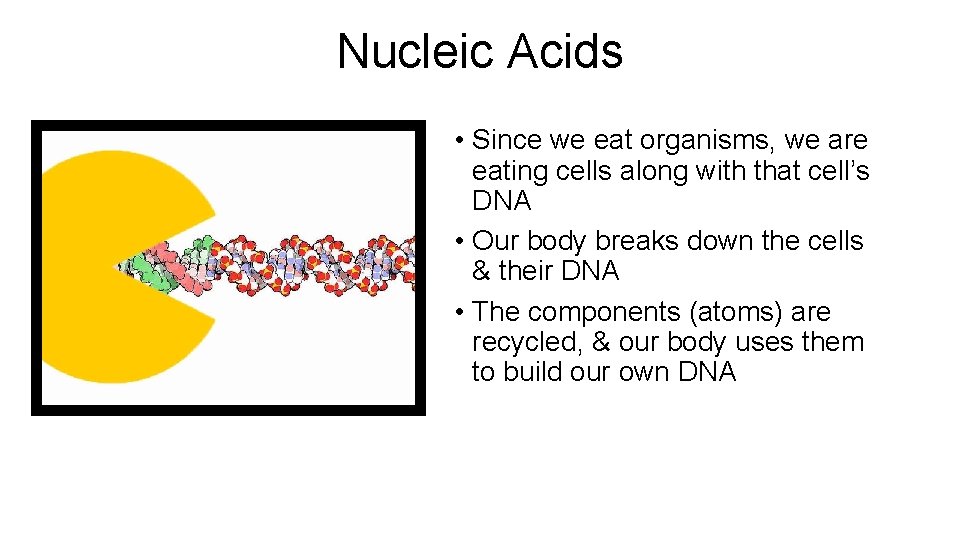
Nucleic Acids • Since we eat organisms, we are eating cells along with that cell’s DNA • Our body breaks down the cells & their DNA • The components (atoms) are recycled, & our body uses them to build our own DNA
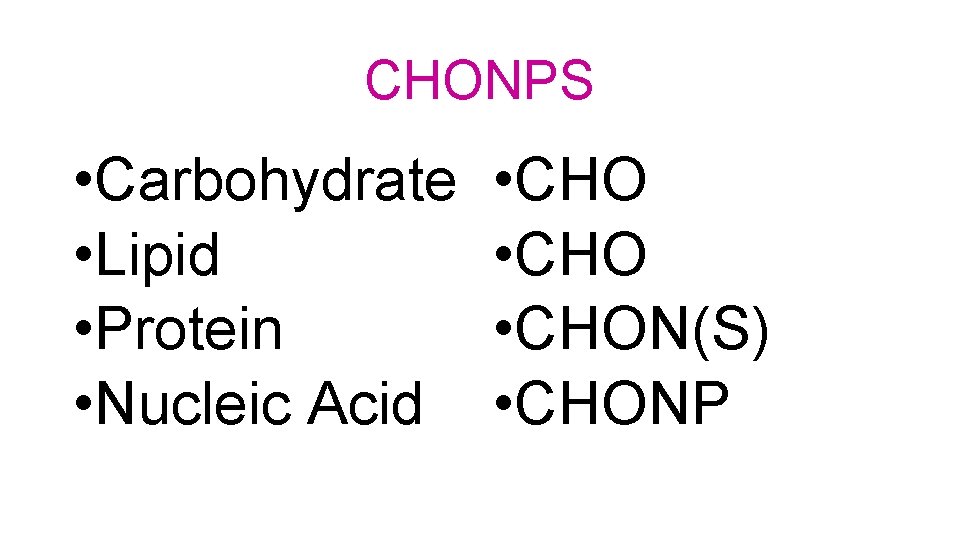
CHONPS • Carbohydrate • Lipid • Protein • Nucleic Acid • CHON(S) • CHONP
- Slides: 15