Proteins Regents Biology Proteins Multipurpose molecules Regents Biology

Proteins Regents Biology
Proteins: Multipurpose molecules Regents Biology 2006 -2007
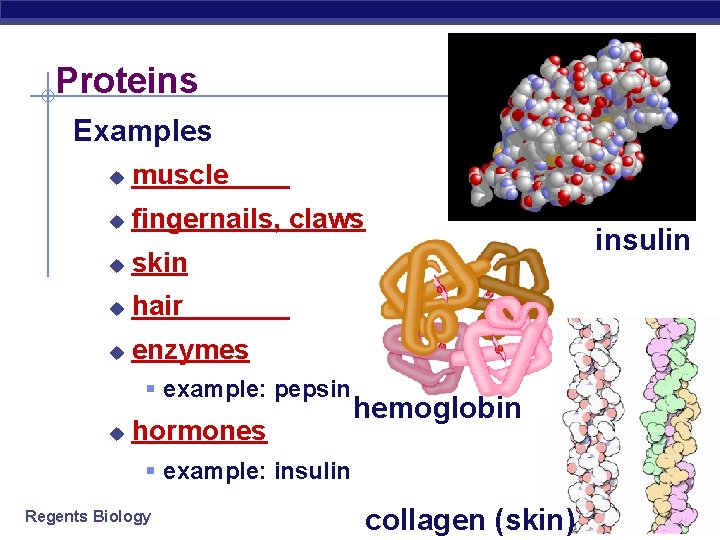
Proteins Examples u muscle u fingernails, claws u skin u hair u enzymes § example: pepsin u hormones insulin hemoglobin § example: insulin Regents Biology collagen (skin)
Proteins § Function: u many, many functions § hormones w insulin § movement w muscle § immune system w protect against germs § enzymes w help chemical reactions Regents Biology

Proteins § Building block = amino acids amino amino acid – acid u 20 different amino acids H O H | || —N— —C—C—OH | H variable Regents Biology group
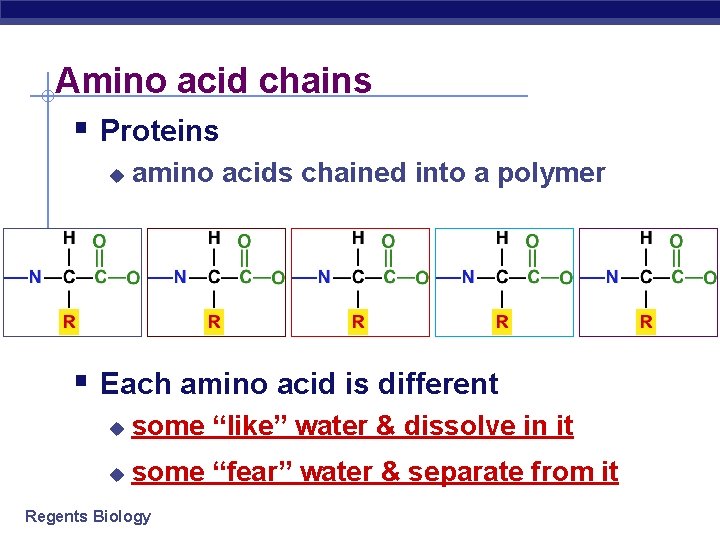
Amino acid chains § Proteins u amino acids chained into a polymer § Each amino acid is different u some “like” water & dissolve in it u some “fear” water & separate from it Regents Biology
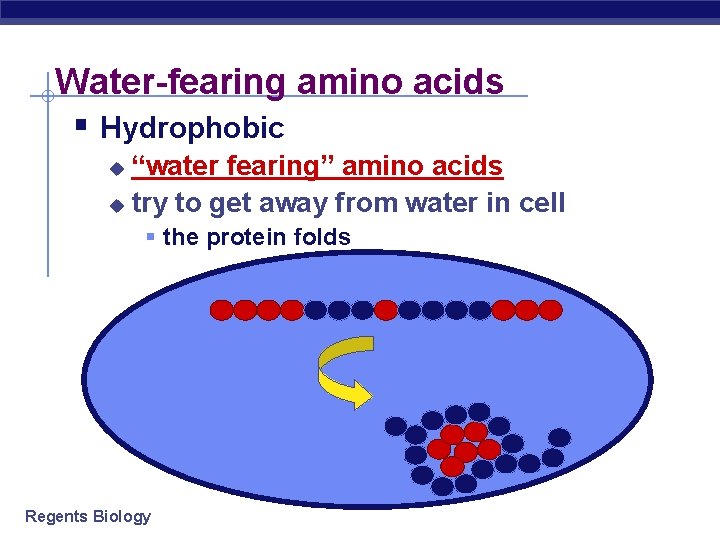
Water-fearing amino acids § Hydrophobic “water fearing” amino acids u try to get away from water in cell u § the protein folds Regents Biology

Water-loving amino acids § Hydrophillic “water loving” amino acids u try to stay in water in cell u § the protein folds Regents Biology

3 -D protein structure § Proteins fold & twist into 3 -D shape hemoglobin growth hormone Regents Biology pepsin collagen
Proteins (Polypeptides) Four levels of protein structure: A. Primary Structure B. Secondary Structure C. Tertiary Structure D. Quaternary Structure Regents Biology
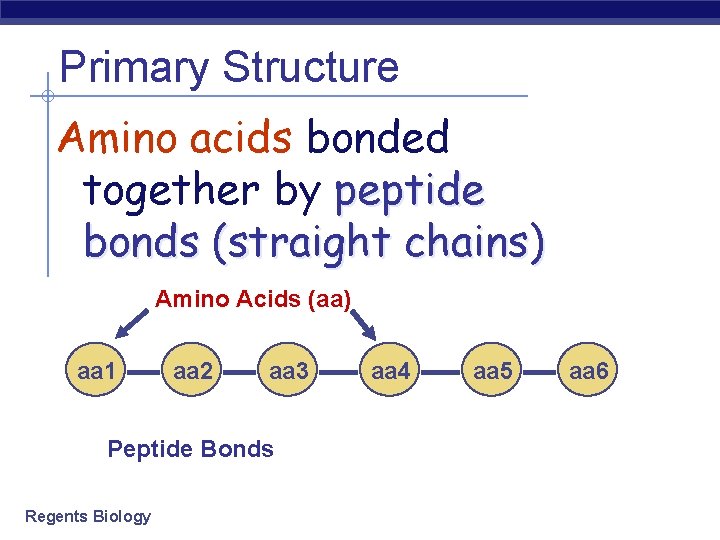
Primary Structure Amino acids bonded together by peptide bonds (straight chains) Amino Acids (aa) aa 1 aa 2 aa 3 Peptide Bonds Regents Biology aa 4 aa 5 aa 6
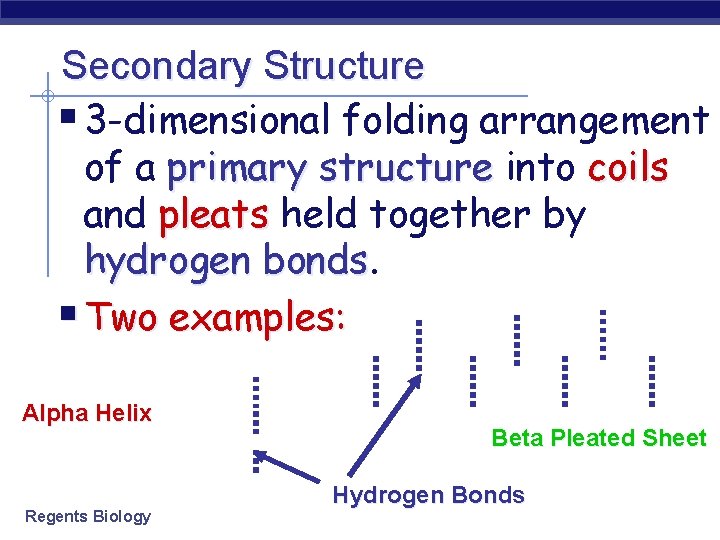
Secondary Structure § 3 -dimensional folding arrangement of a primary structure into coils and pleats held together by hydrogen bonds § Two examples: Alpha Helix Regents Biology Beta Pleated Sheet Hydrogen Bonds

Tertiary Structure § Secondary structures bent and folded into a more complex 3 -D arrangement of linked polypeptides § Bonds: H-bonds, ionic, disulfide bridges (S-S) § Call a “subunit”. Alpha Helix Regents Biology Beta Pleated Sheet
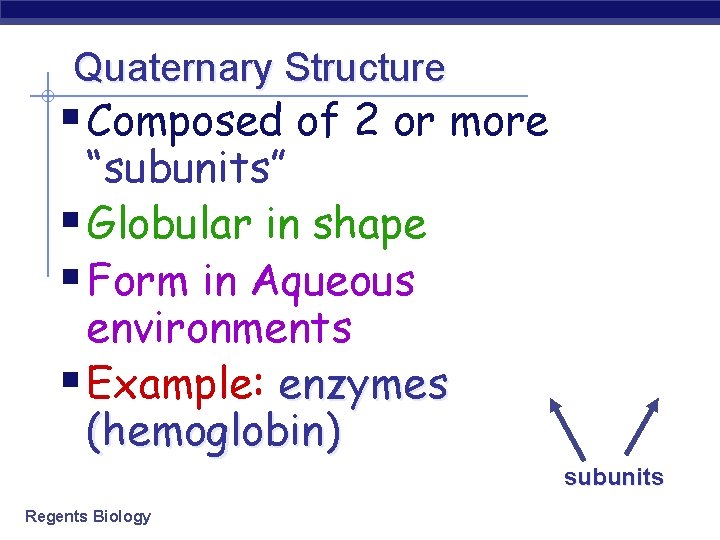
Quaternary Structure § Composed of 2 or more “subunits” § Globular in shape § Form in Aqueous environments § Example: enzymes (hemoglobin) subunits Regents Biology

Its shape that matters! § Proteins do their jobs, because § of their shape Unfolding a protein destroys its shape wrong shape = can’t do its job u unfolding proteins = “denature” u § temperature § p. H (acidity) folded Regents Biology unfolded “denatured”
- Slides: 15