PROTEINS Building Blocks of Life PROTEINS Most cellular
PROTEINS Building Blocks of Life

PROTEINS Most cellular structures are made up of various types of protein Structural building blocks Extremely diverse Make up 50% of dry mass in cells Examples: hair, nails, muscle tissue, enzymes, bone, cartilage, hormones, nerve tissues, etc. Diverse functions depend on linkage of amino acids (a. a. )
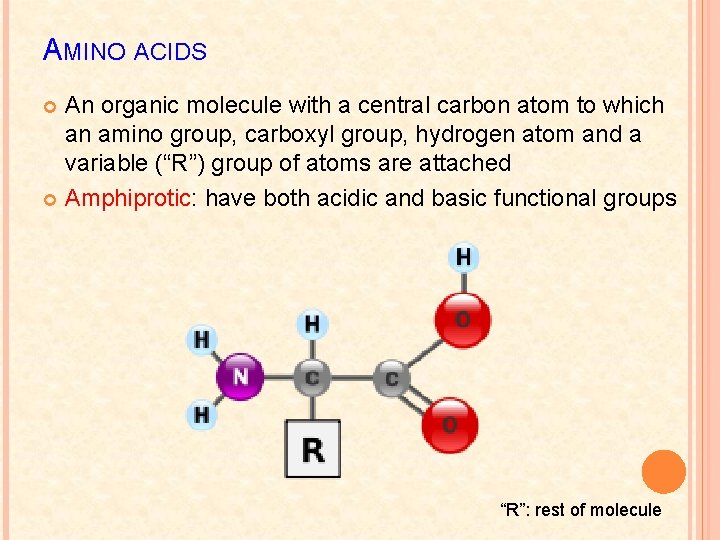
AMINO ACIDS An organic molecule with a central carbon atom to which an amino group, carboxyl group, hydrogen atom and a variable (“R”) group of atoms are attached Amphiprotic: have both acidic and basic functional groups “R”: rest of molecule

AMINO ACIDS 20 different amino acids 8 are essential: your body cannot synthesize them, get them through diet only Can be polar, non-polar, charged – all depends on the nature of their side chain or “R” group When dissolved in water, the carboxyl group donates an H+ ion to the amino group � Carboxyl side becomes negatively charged � Amino side becomes positively charged Pg. 39 Fig 1

Pg. 40 Fig 2
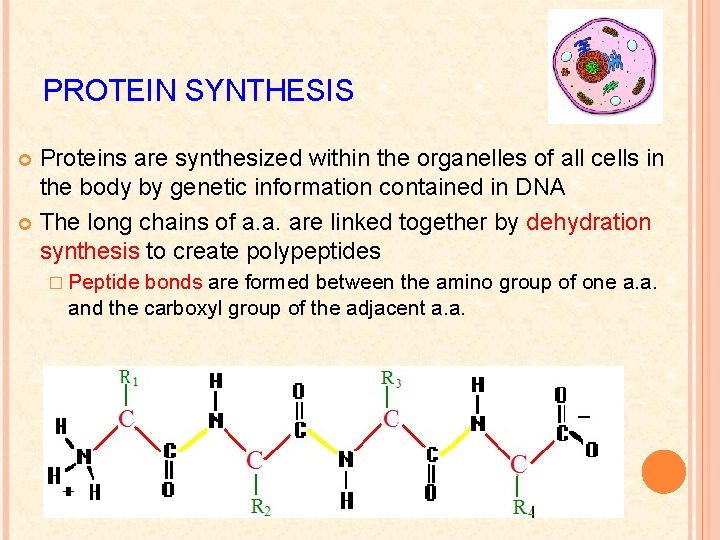
PROTEIN SYNTHESIS Proteins are synthesized within the organelles of all cells in the body by genetic information contained in DNA The long chains of a. a. are linked together by dehydration synthesis to create polypeptides � Peptide bonds are formed between the amino group of one a. a. and the carboxyl group of the adjacent a. a.

Dehydration Synthesis Example: Glycine + Glycine (Gly) + H 2 O
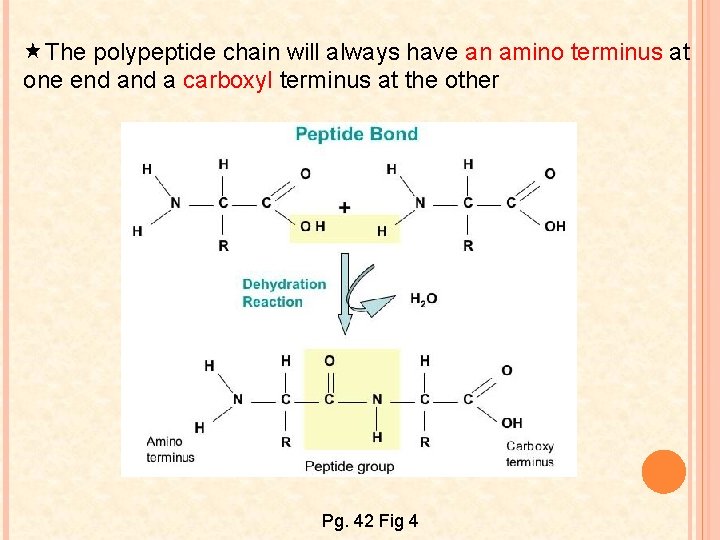
The polypeptide chain will always have an amino terminus at one end a carboxyl terminus at the other Pg. 42 Fig 4

THE SEQUENCE OF AMINO ACIDS IN A PROTEIN DETERMINES THE FUNCTION OF THAT PROTEIN
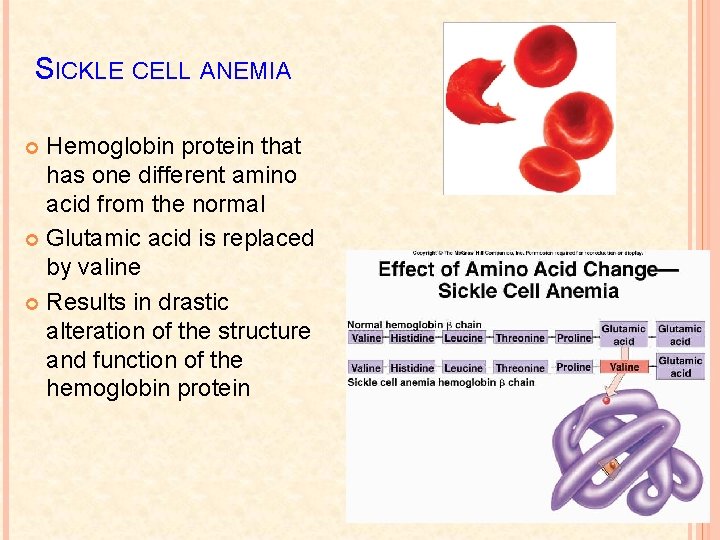
SICKLE CELL ANEMIA Hemoglobin protein that has one different amino acid from the normal Glutamic acid is replaced by valine Results in drastic alteration of the structure and function of the hemoglobin protein
PROTEIN STRUCTURE Proteins may be described in 4 levels of structure 1. Primary Structure 2. Secondary Structure 3. Tertiary Structure 4. Quaternary Structure
PRIMARY STRUCTURE Unique sequence of amino acids in a polypeptide chain Each amino acid is called a “residue” The DNA governs the order in which the individual amino acids are linked to form the chain Most important structure! The order of amino acids determines the type of protein that is built. If the sequence changes even by one a. a. the 3 D structure could be altered.

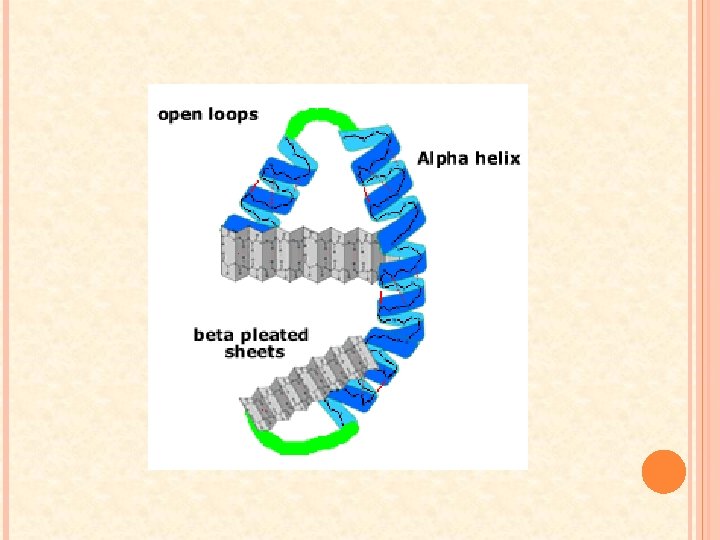
SECONDARY STRUCTURE As the a. a. ’s are added to the polypeptide chain, it coils and folds at various locations along its length Two types: 1. α-helix: � At certain regions of the polypeptide a hydrogen bond forms between the carboxyl group of one peptide and the amino group four peptide bonds away � These hydrogen bonds are repeated over a certain length of the chain shaping portions of the polypeptide into a coil


2. Β-pleated sheets � When two parts of the polypeptide chain lie parallel to one another, hydrogen bonds between the oxygen atoms of the carboxyl group on one strand hydrogen atoms from the amino group on the adjacent strain

TERTIARY STRUCTURE Strong forces of attraction and repulsion between the polypeptide and its environment forces it to undergo additional folding a. a. with hydrophobic groups are placed in the interior of the folded polypeptide – away from water Stabilized by a number of R group interactions 1. 2. Ionic bonds and Van der Waals forces keep the polypeptide folded When sulfur containing R groups are brought close together a covalent bond called a disulfide bridge may form
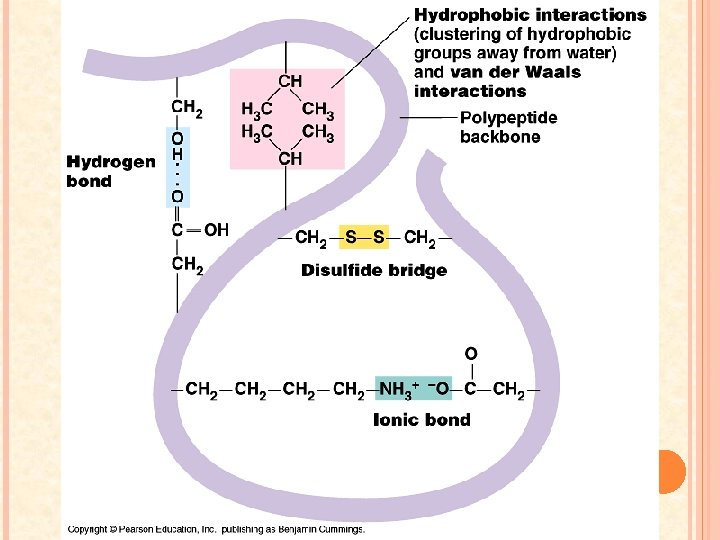


QUATERNARY STRUCTURE Not all proteins need this structure In some cases 2 or more polypeptide units must come together to form a functional protein Examples: collagen, keratin, hemoglobin
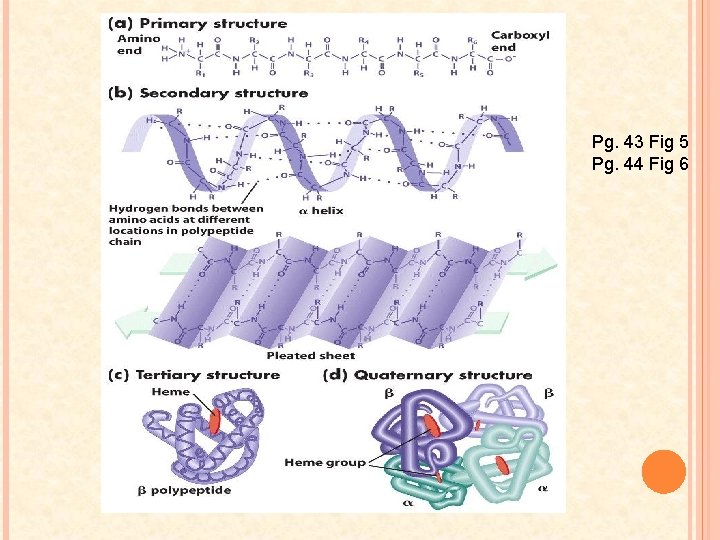
Pg. 43 Fig 5 Pg. 44 Fig 6

PROTEIN DENATURATION Change in the 3 D shape of a protein caused by changes in temperature, p. H, etc is called denaturation Causes the protein to lose its tertiary or quaternary shape by disrupting the bonds of the protein structure A denatured protein cannot carry out its function However, it will usually return to its normal shape and function (as long as the primary structure remains intact) once the denaturing agent has been removed Ex. straightening hair


USES AND DANGERS Uses: � Style hair � Curing meats by denaturing protein that would cause them to brown Dangers � Prolonged fever could denature critical enzymes in brain � If primary structure is damaged, protein can no longer return to its normal shape: protein considered dead

HOMEWORK Section 1. 5 p. 39 -44 Answer p. 47 #1 -7 Answer SG p. 13 #1 -5 Protein Worksheet *can be done tmr during class Upcoming Dates: Fri: Quiz (carbs/proteins) Fri Mar 1: Unit 1 Test Tues Mar 5: Project
- Slides: 25