Proteins are macromolecules made up of amino acids

- Slides: 19

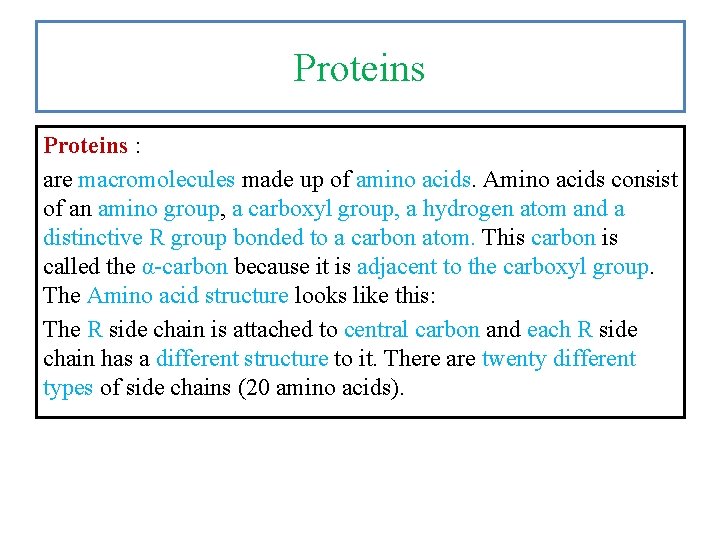
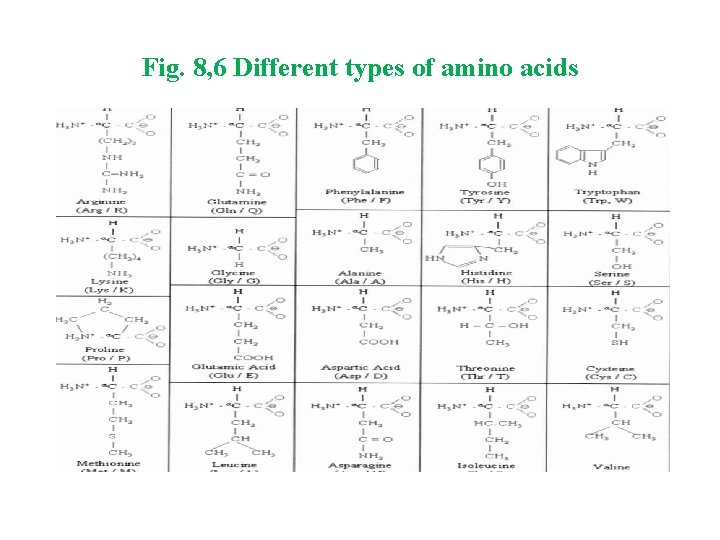
Proteins : are macromolecules made up of amino acids. Amino acids consist of an amino group, a carboxyl group, a hydrogen atom and a distinctive R group bonded to a carbon atom. This carbon is called the α-carbon because it is adjacent to the carboxyl group. The Amino acid structure looks like this: The R side chain is attached to central carbon and each R side chain has a different structure to it. There are twenty different types of side chains (20 amino acids).

Fig. 8. 4 General structure of amino acid

Peptides and Proteins Amino acids are linked together to form long chains. They are linked together by peptide bonds a special covalent bond found in proteins (sometimes called Polypeptides). After the chains of Amino acids are constructed, they can fold into more complex structures.

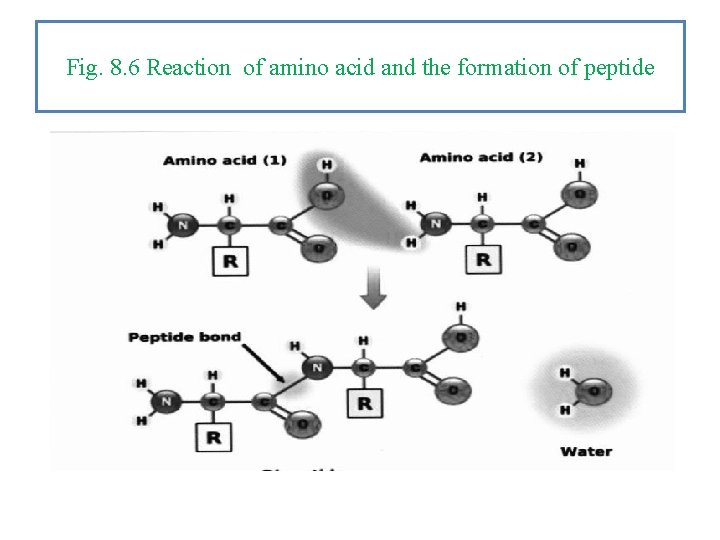
Fig. 8. 6 Reaction of amino acid and the formation of peptide

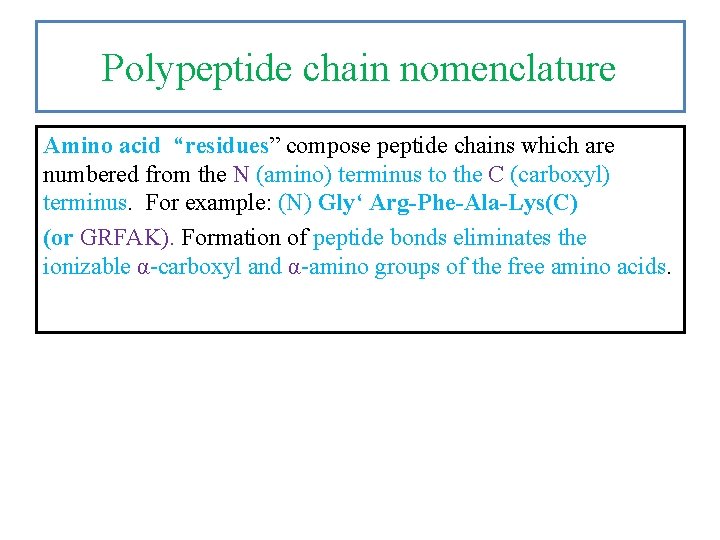
Polypeptide chain nomenclature Amino acid “residues” compose peptide chains which are numbered from the N (amino) terminus to the C (carboxyl) terminus. For example: (N) Gly‘ Arg-Phe-Ala-Lys(C) (or GRFAK). Formation of peptide bonds eliminates the ionizable α-carboxyl and α-amino groups of the free amino acids.

Fig. 8, 6 Different types of amino acids

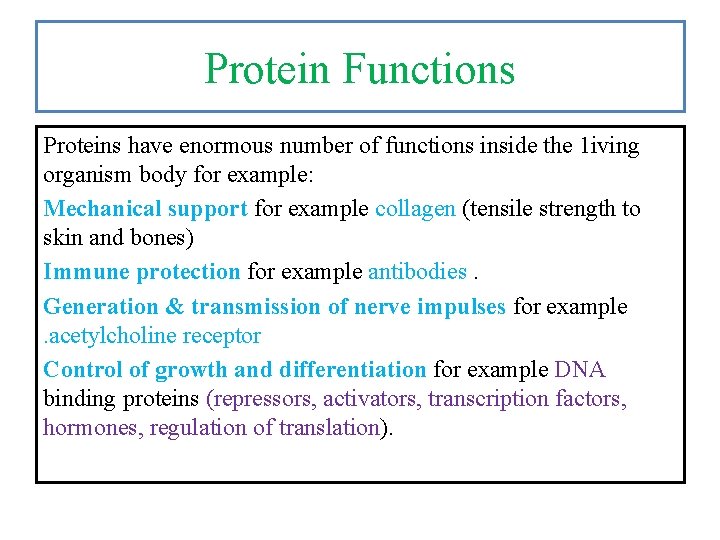
Protein Functions Proteins have enormous number of functions inside the 1 iving organism body for example: Mechanical support for example collagen (tensile strength to skin and bones) Immune protection for example antibodies. Generation & transmission of nerve impulses for example. acetylcholine receptor Control of growth and differentiation for example DNA binding proteins (repressors, activators, transcription factors, hormones, regulation of translation).

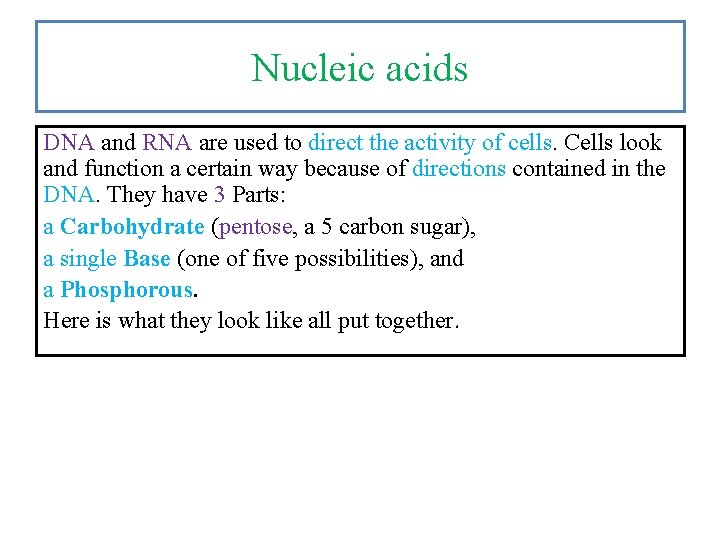
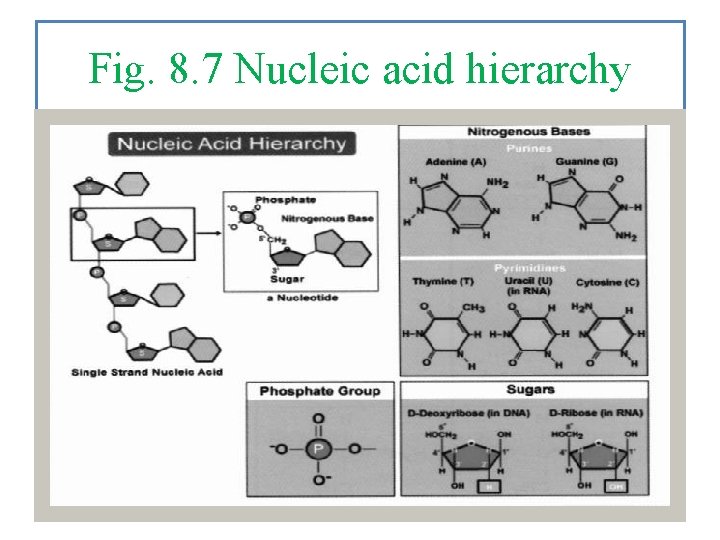
Nucleic acids DNA and RNA are used to direct the activity of cells. Cells look and function a certain way because of directions contained in the DNA. They have 3 Parts: a Carbohydrate (pentose, a 5 carbon sugar), a single Base (one of five possibilities), and a Phosphorous. Here is what they look like all put together.

Fig. 8. 7 Nucleic acid hierarchy

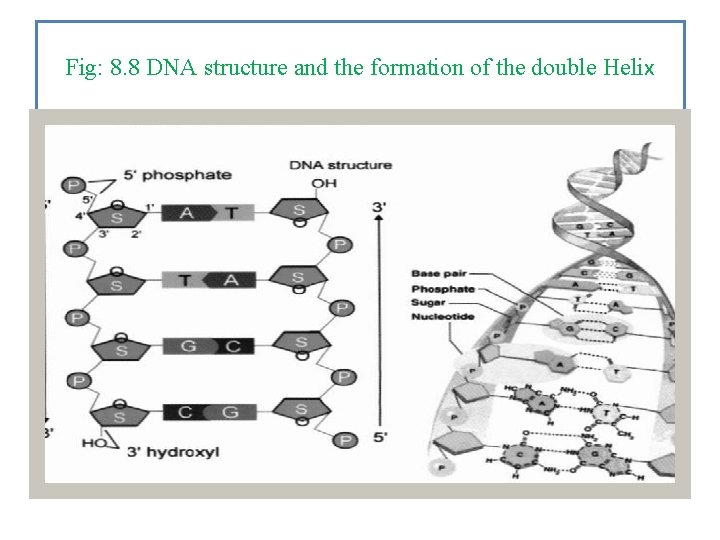
Nucleic acids are linked together to form long chains, and DNA is made of two parallel chains. These parallel chains have a twist to them so DNA is often called a Double Hellx making the DNA molecules as shown in Fig. 8. 8.

Fig: 8. 8 DNA structure and the formation of the double Helix

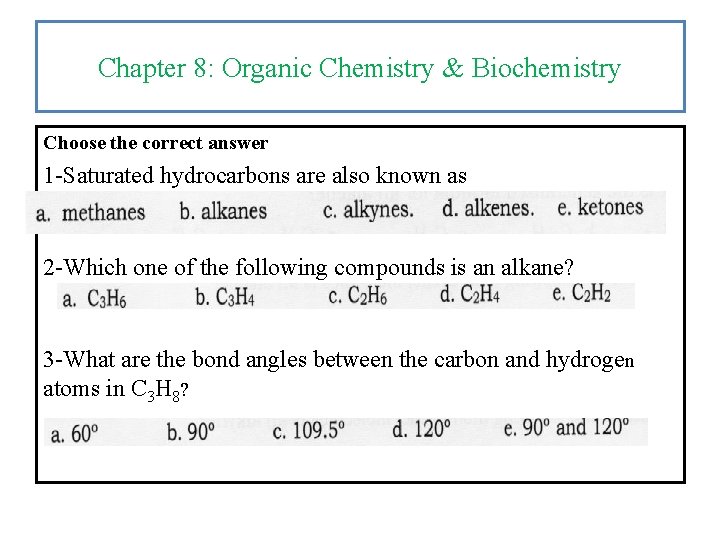
Chapter 8: Organic Chemistry & Biochemistry Choose the correct answer 1 -Saturated hydrocarbons are also known as 2 -Which one of the following compounds is an alkane? 3 -What are the bond angles between the carbon and hydrogen atoms in C 3 H 8?

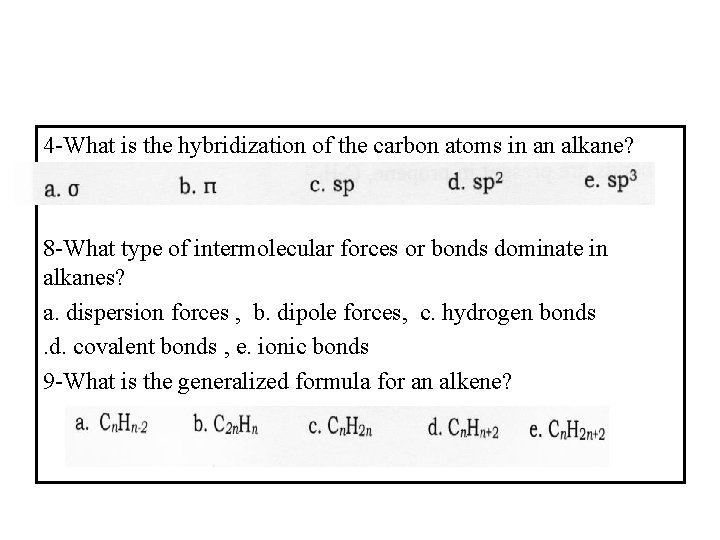
4 -What is the hybridization of the carbon atoms in an alkane? 8 -What type of intermolecular forces or bonds dominate in alkanes? a. dispersion forces , b. dipole forces, c. hydrogen bonds. d. covalent bonds , e. ionic bonds 9 -What is the generalized formula for an alkene?

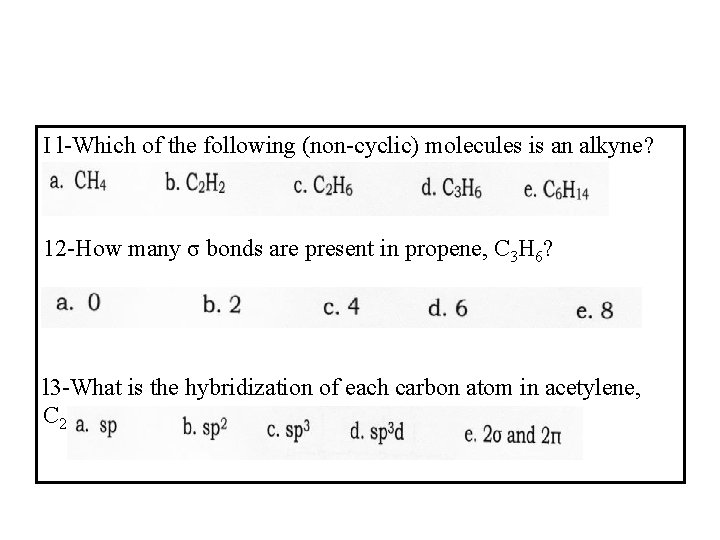
I l-Which of the following (non-cyclic) molecules is an alkyne? 12 -How many σ bonds are present in propene, C 3 H 6? l 3 -What is the hybridization of each carbon atom in acetylene, C 2 H 2?

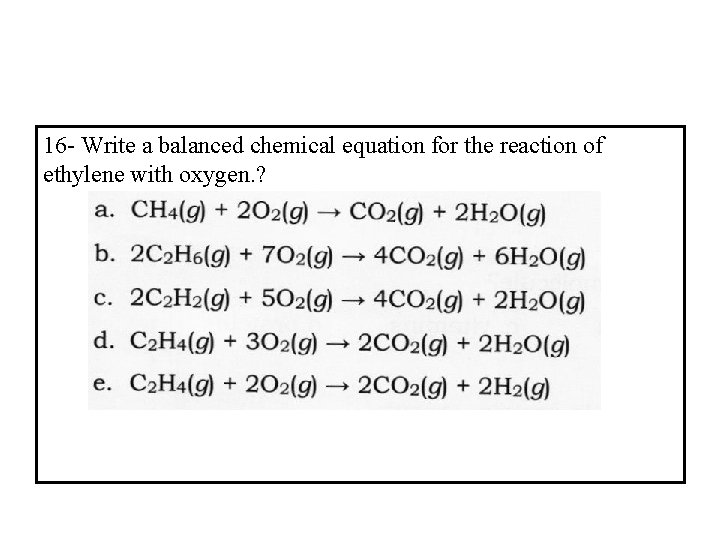
16 - Write a balanced chemical equation for the reaction of ethylene with oxygen. ?

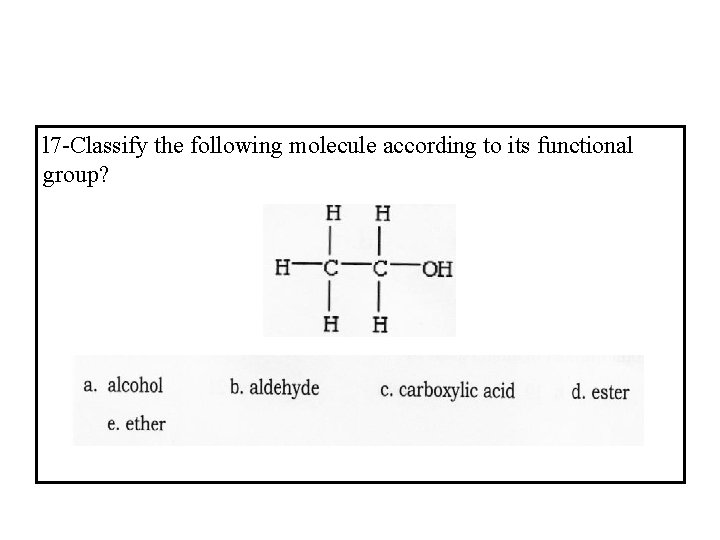
l 7 -Classify the following molecule according to its functional group?

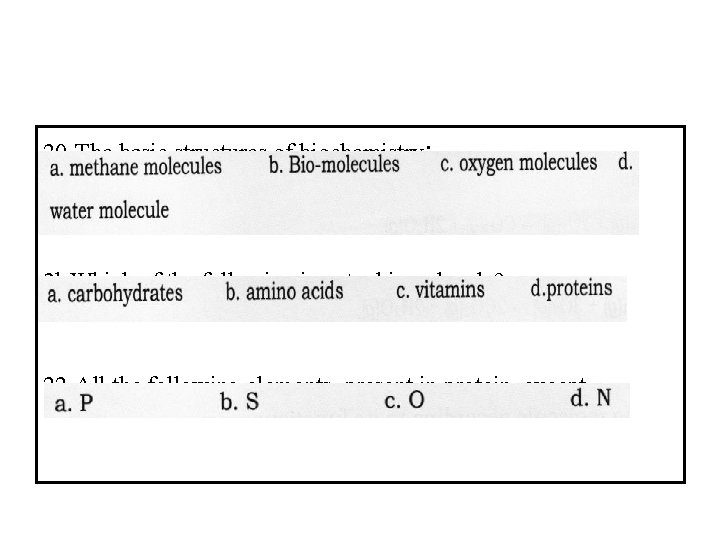
20 -The basic structures of biochemistry: 2 l-Which of the following is not a biomolecule? 22 -All the following elements, present in protein, except

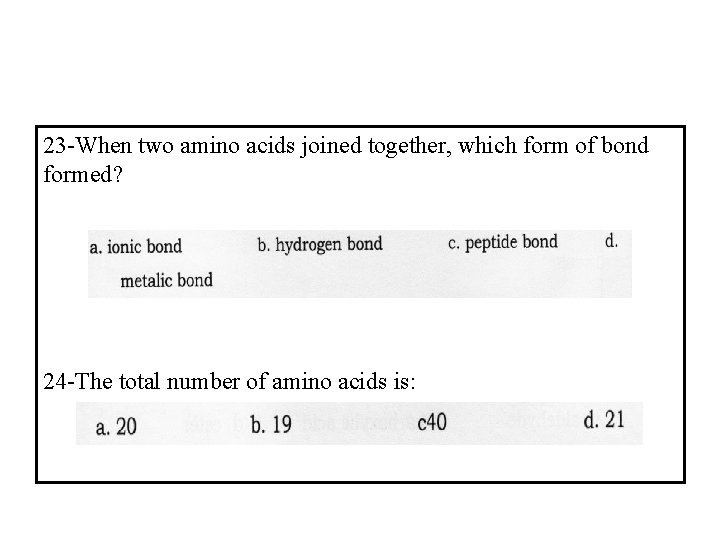
23 -When two amino acids joined together, which form of bond formed? 24 -The total number of amino acids is:

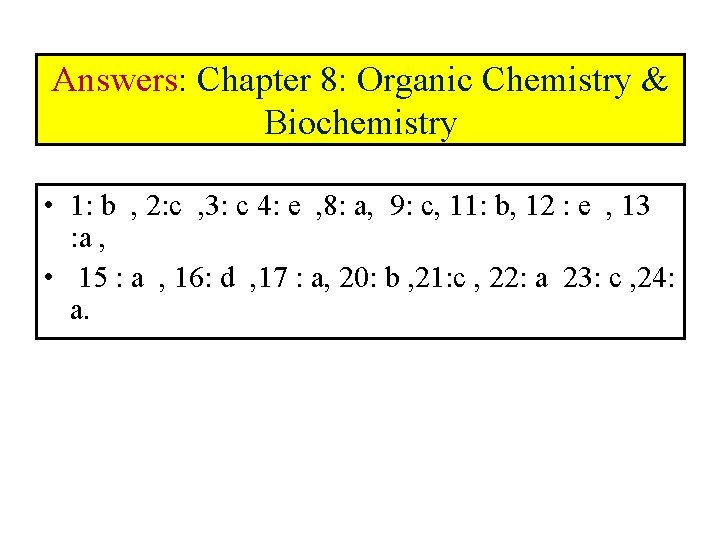
Answers: Chapter 8: Organic Chemistry & Biochemistry • 1: b , 2: c , 3: c 4: e , 8: a, 9: c, 11: b, 12 : e , 13 : a , • 15 : a , 16: d , 17 : a, 20: b , 21: c , 22: a 23: c , 24: a.