Proteins AP Biology Proteins Multipurpose molecules AP Biology

Proteins AP Biology
Proteins Multipurpose molecules AP Biology 2006 -2007
GENERAL CHARACTERISTICS & IMPORTANCES: Polymers of amino acids Each has unique 3 -D shape Vary in sequence of amino acids Major component of cell parts Provide support Storage of amino acids Receptor proteins; contractile proteins; antibodies; enzymes AP Biology
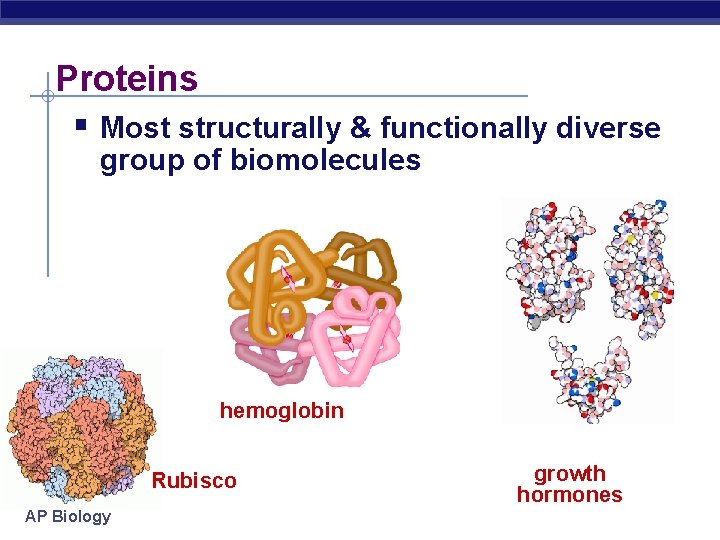
Proteins Most structurally & functionally diverse group of biomolecules hemoglobin Rubisco AP Biology growth hormones
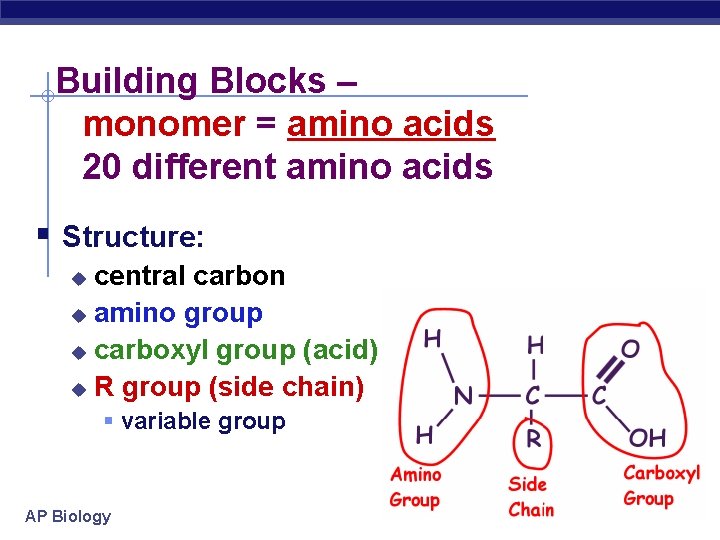
Building Blocks – monomer = amino acids 20 different amino acids Structure: central carbon amino group carboxyl group (acid) R group (side chain) variable group AP Biology
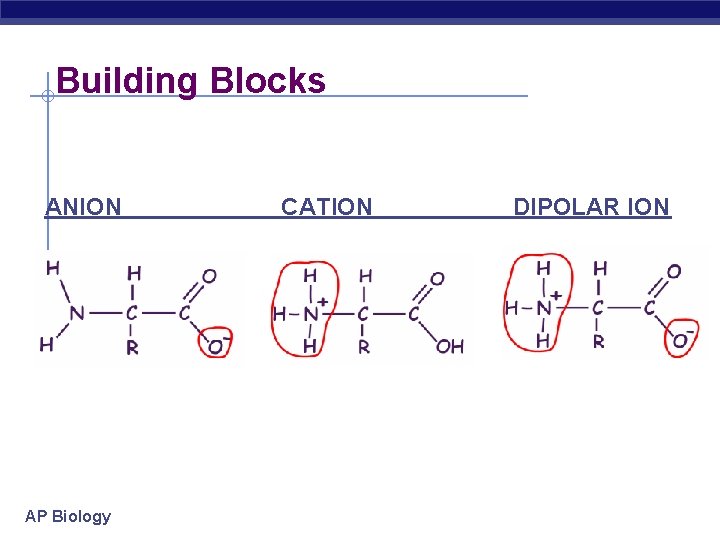
Building Blocks ANION AP Biology CATION DIPOLAR ION
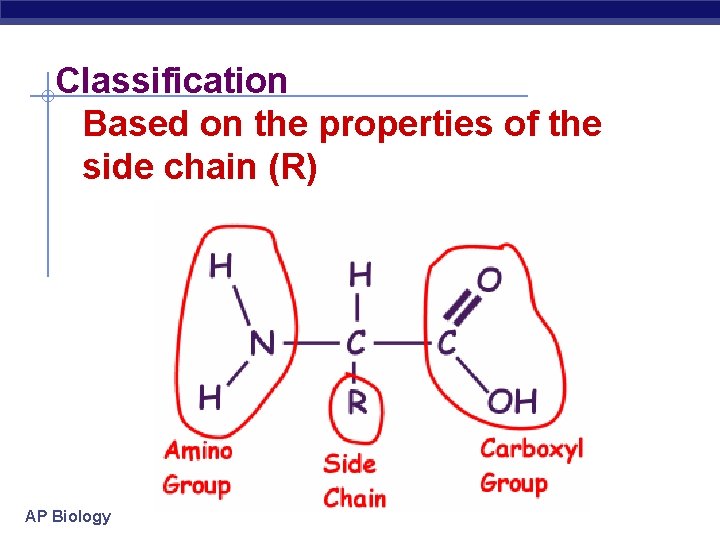
Classification Based on the properties of the side chain (R) AP Biology

Nonpolar amino acids Hydrocarbon chains No oxygen AP Biology
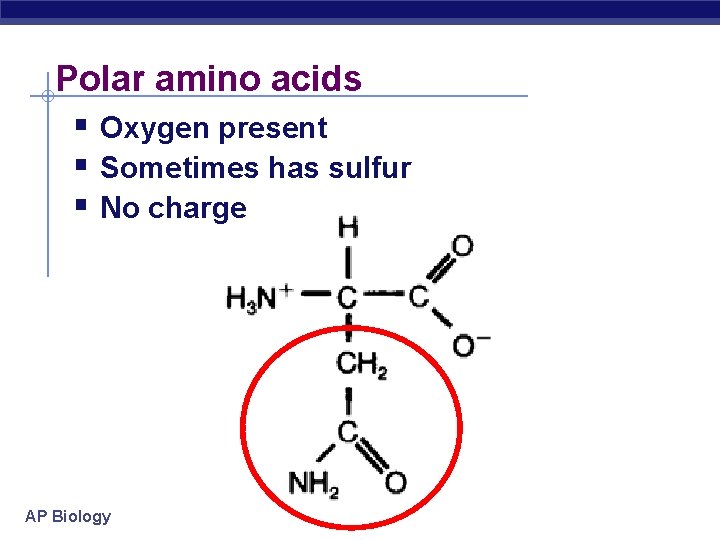
Polar amino acids Oxygen present Sometimes has sulfur No charge AP Biology
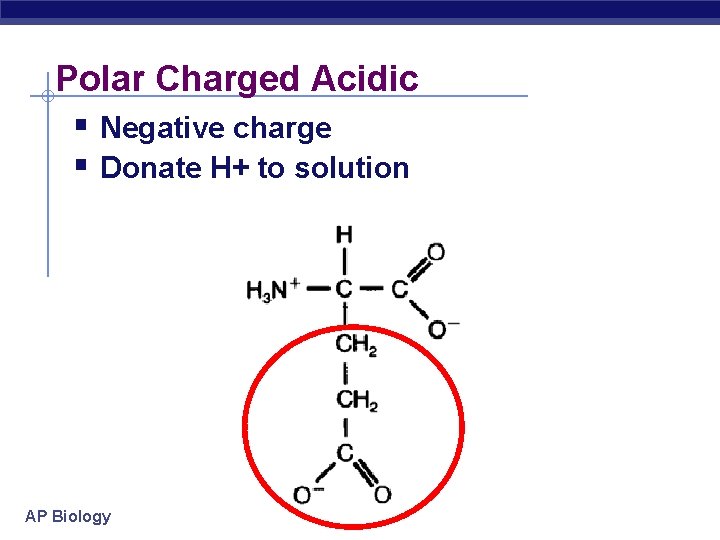
Polar Charged Acidic Negative charge Donate H+ to solution AP Biology
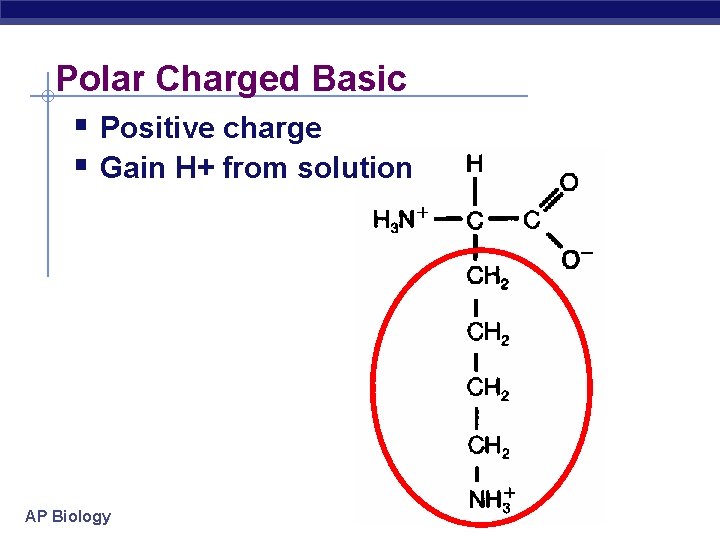
Polar Charged Basic Positive charge Gain H+ from solution AP Biology

Nonpolar amino acids nonpolar & hydrophobic AP Biology
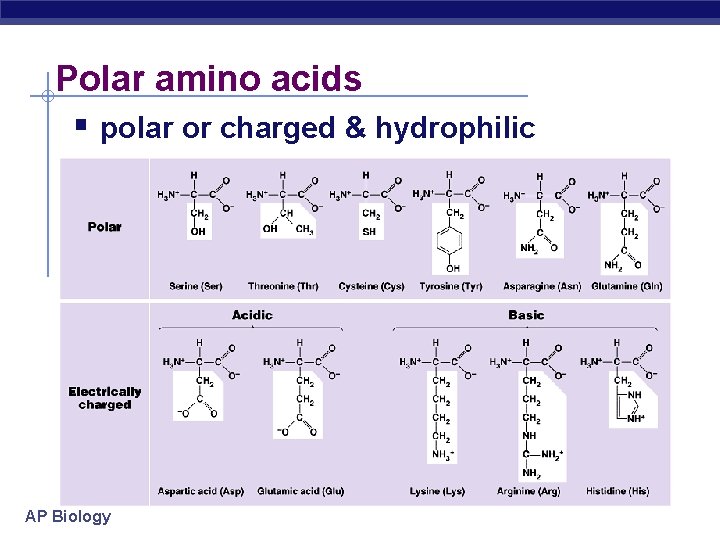
Polar amino acids polar or charged & hydrophilic AP Biology
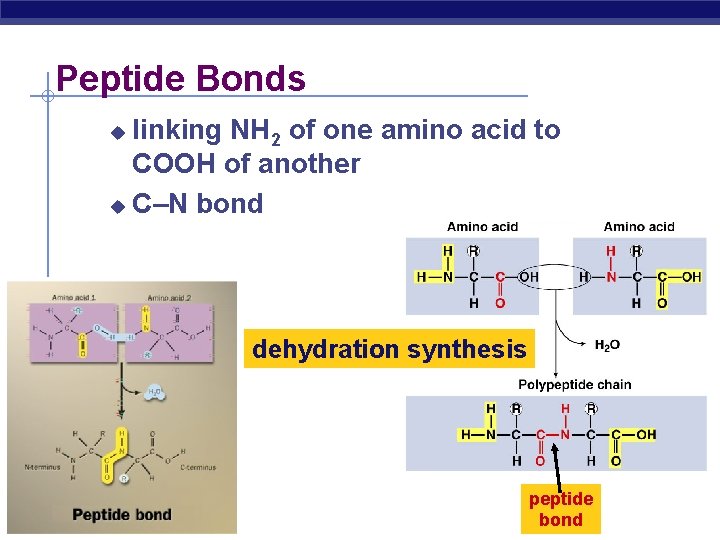
Peptide Bonds linking NH 2 of one amino acid to COOH of another C–N bond dehydration synthesis AP Biology peptide bond
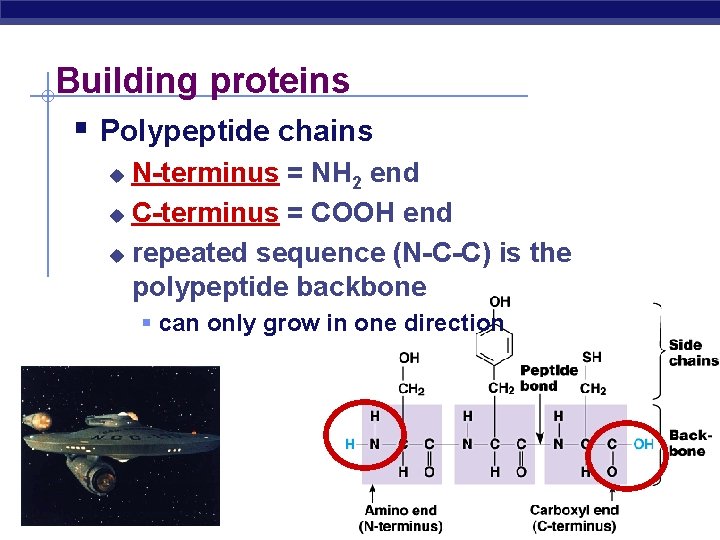
Building proteins Polypeptide chains N-terminus = NH 2 end C-terminus = COOH end repeated sequence (N-C-C) is the polypeptide backbone can only grow in one direction AP Biology
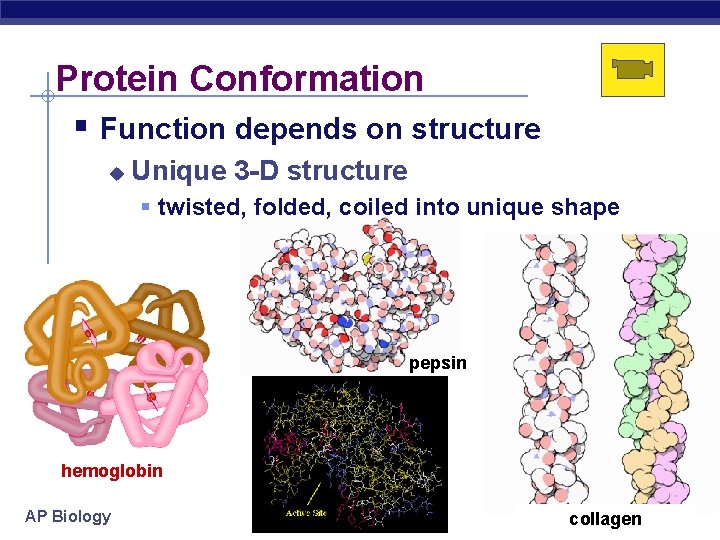
Protein Conformation Function depends on structure Unique 3 -D structure twisted, folded, coiled into unique shape pepsin hemoglobin AP Biology collagen

Primary (1°) structure Order of amino acids in chain amino acid sequence determined by gene (DNA) slight change in amino acid sequence can affect protein’s structure & it’s function even just one amino acid change can make all the difference! AP Biology lysozyme: enzyme in tears & mucus that kills bacteria
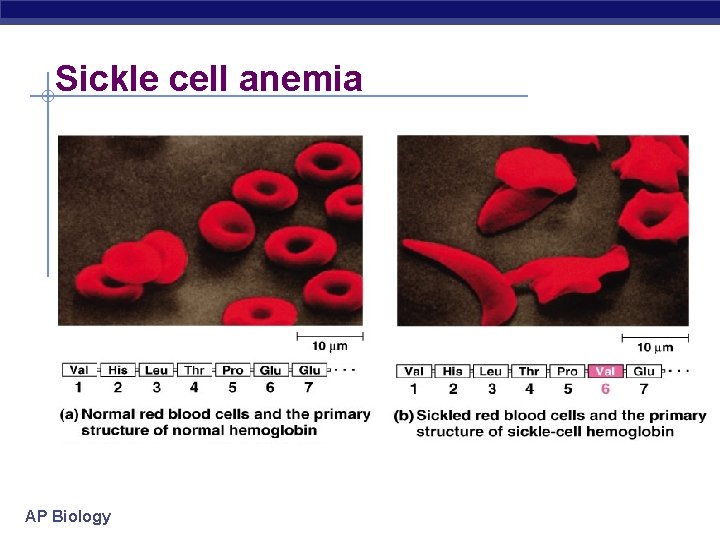
Sickle cell anemia AP Biology
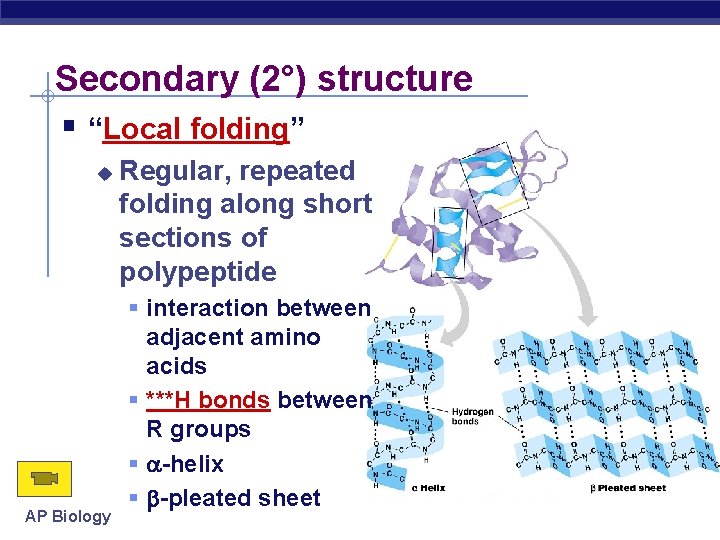
Secondary (2°) structure “Local folding” AP Biology Regular, repeated folding along short sections of polypeptide interaction between adjacent amino acids ***H bonds between R groups -helix -pleated sheet
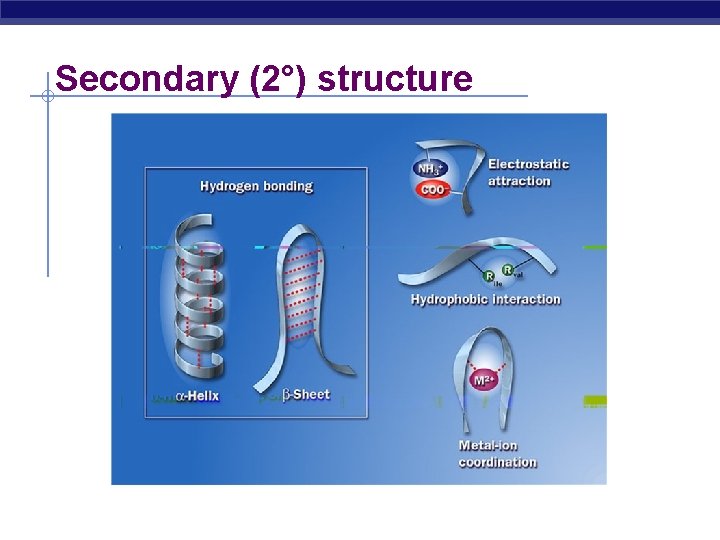
Secondary (2°) structure AP Biology

Tertiary (3°) structure (Globular) “Unique 3 -D shape of fully folded polypeptide” (Whole molecule folding) determined by interactions between R groups AP Biology hydrophobic interactions w effect of water in cell anchored by disulfide bridges (H & ionic bonds)
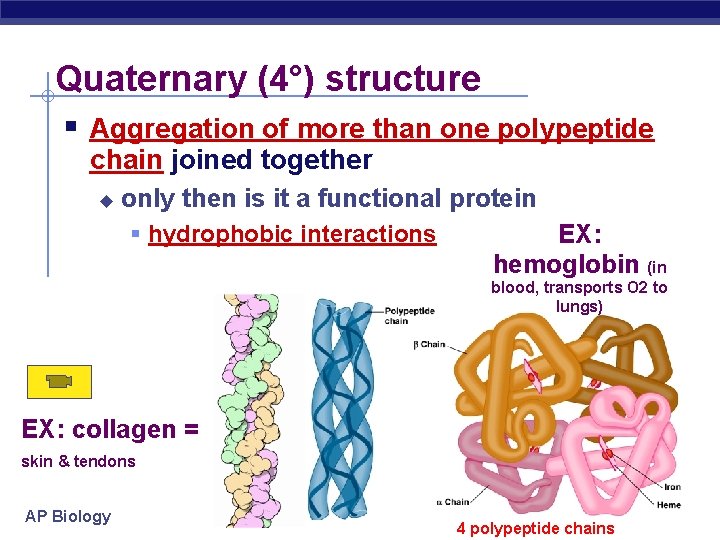
Quaternary (4°) structure Aggregation of more than one polypeptide chain joined together only then is it a functional protein hydrophobic interactions EX: hemoglobin (in blood, transports O 2 to lungs) EX: collagen = skin & tendons AP Biology 4 polypeptide chains
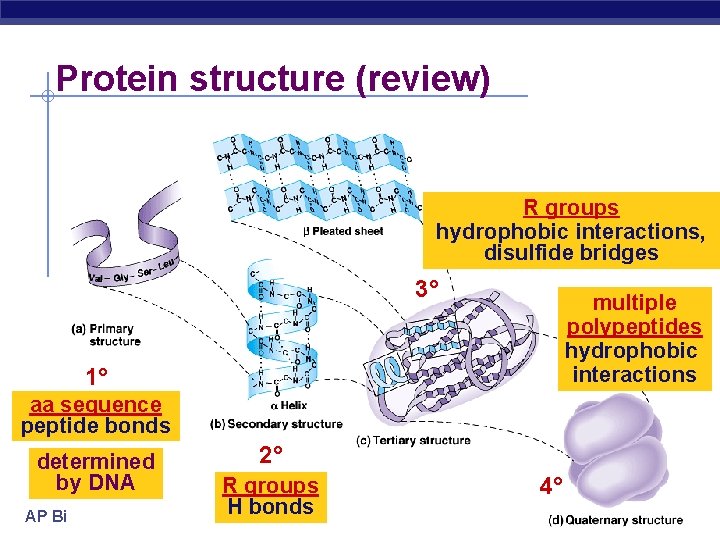
Protein structure (review) R groups hydrophobic interactions, disulfide bridges 3° multiple polypeptides hydrophobic interactions 1° aa sequence peptide bonds determined by DNA AP Biology 2° R groups H bonds 4°
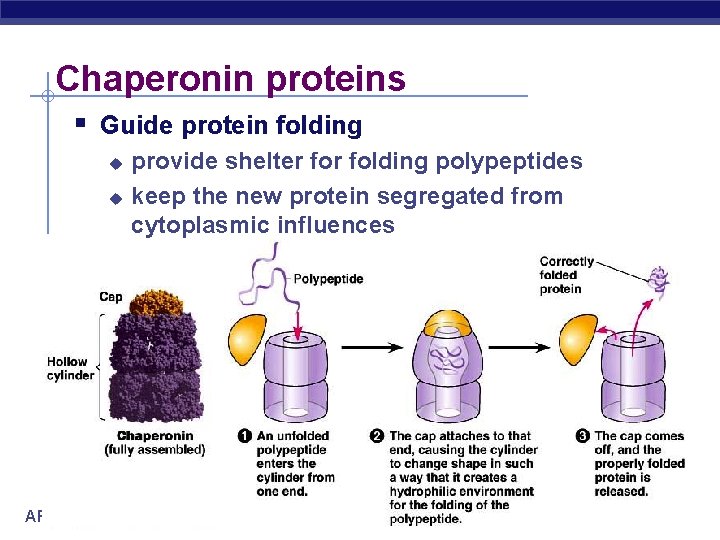
Chaperonin proteins Guide protein folding AP Biology provide shelter folding polypeptides keep the new protein segregated from cytoplasmic influences
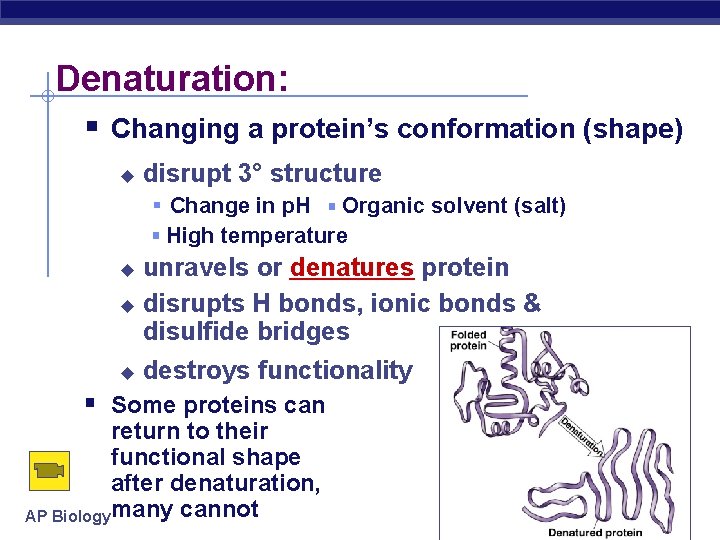
Denaturation: Changing a protein’s conformation (shape) disrupt 3° structure Change in p. H Organic solvent (salt) High temperature unravels or denatures protein disrupts H bonds, ionic bonds & disulfide bridges destroys functionality Some proteins can return to their functional shape after denaturation, AP Biology many cannot
- Slides: 25