Protein Therapeutics a summary and pharmacological classification Benjamin

Protein Therapeutics: a summary and pharmacological classification Benjamin Leader, Quentin J. Baca and David E. Golan Yiben Wang 11/16/11
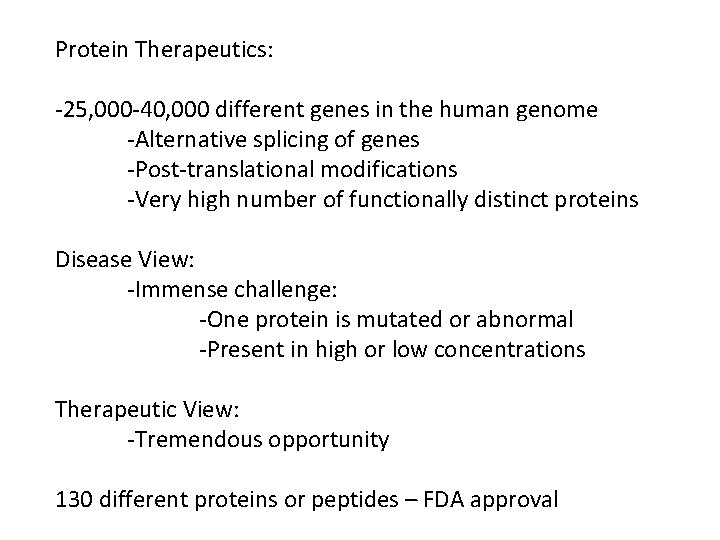
Protein Therapeutics: -25, 000 -40, 000 different genes in the human genome -Alternative splicing of genes -Post-translational modifications -Very high number of functionally distinct proteins Disease View: -Immense challenge: -One protein is mutated or abnormal -Present in high or low concentrations Therapeutic View: -Tremendous opportunity 130 different proteins or peptides – FDA approval
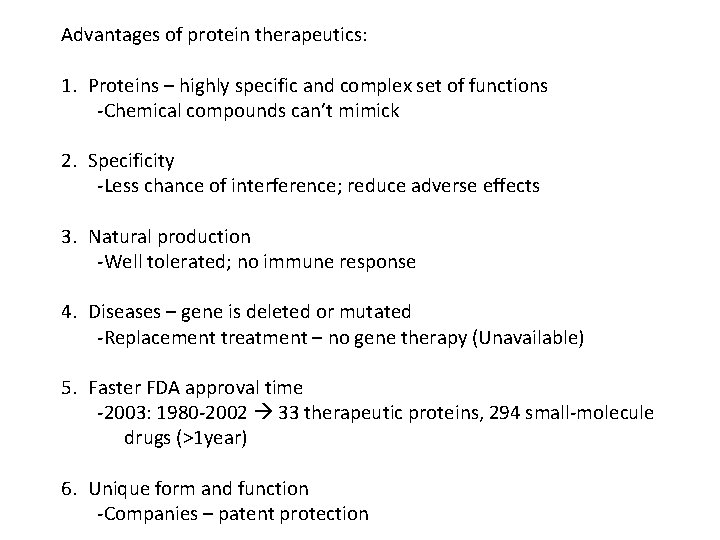
Advantages of protein therapeutics: 1. Proteins – highly specific and complex set of functions -Chemical compounds can’t mimick 2. Specificity -Less chance of interference; reduce adverse effects 3. Natural production -Well tolerated; no immune response 4. Diseases – gene is deleted or mutated -Replacement treatment – no gene therapy (Unavailable) 5. Faster FDA approval time -2003: 1980 -2002 33 therapeutic proteins, 294 small-molecule drugs (>1 year) 6. Unique form and function -Companies – patent protection
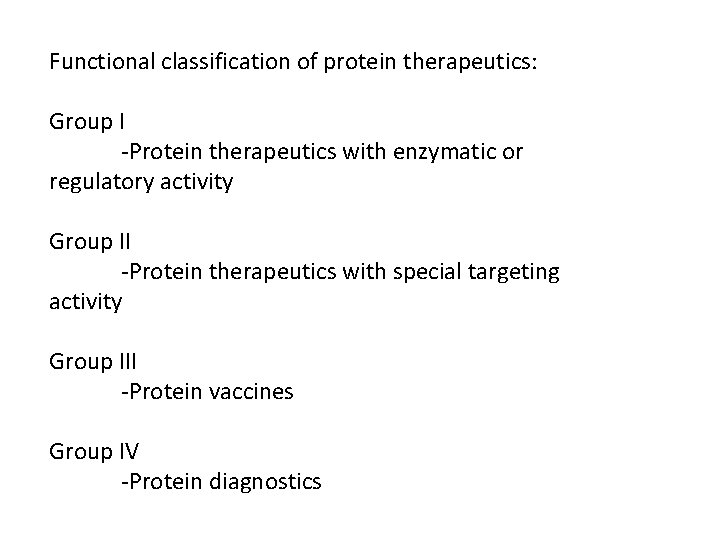
Functional classification of protein therapeutics: Group I -Protein therapeutics with enzymatic or regulatory activity Group II -Protein therapeutics with special targeting activity Group III -Protein vaccines Group IV -Protein diagnostics
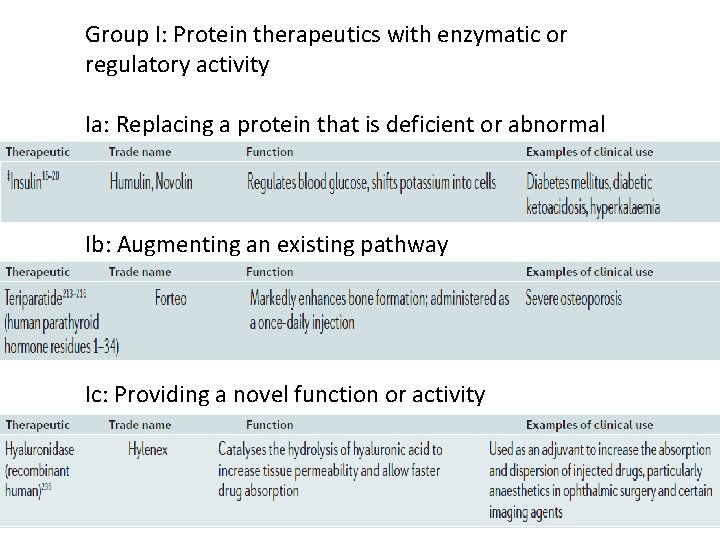
Group I: Protein therapeutics with enzymatic or regulatory activity Ia: Replacing a protein that is deficient or abnormal Ib: Augmenting an existing pathway Ic: Providing a novel function or activity
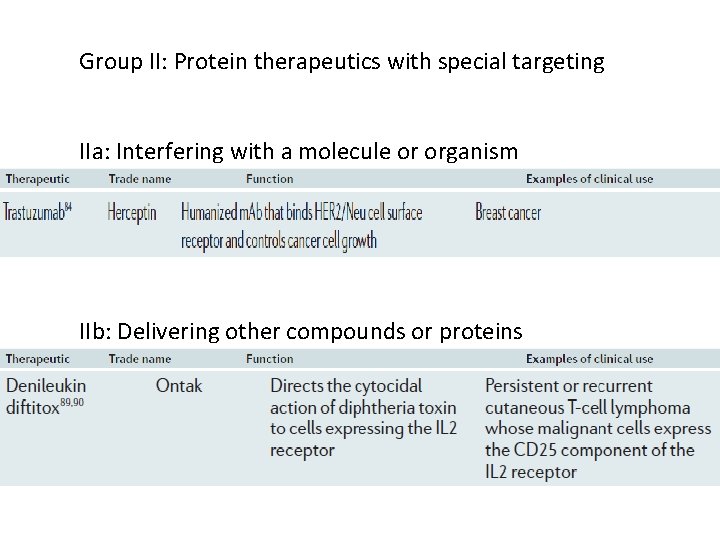
Group II: Protein therapeutics with special targeting IIa: Interfering with a molecule or organism IIb: Delivering other compounds or proteins
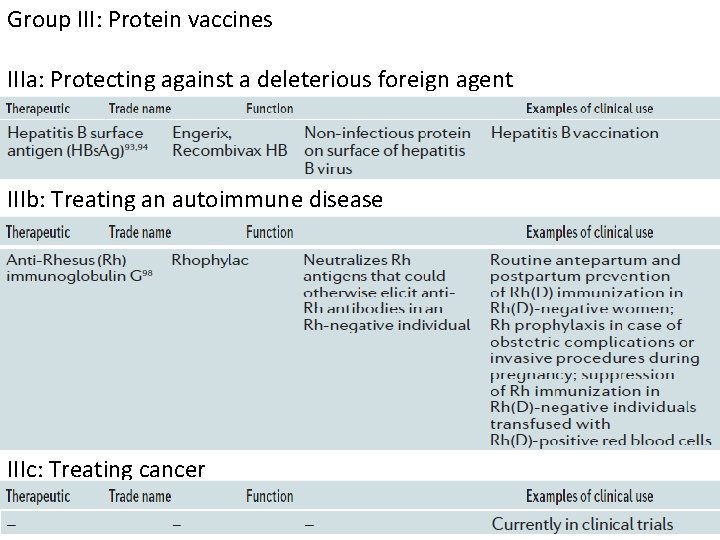
Group III: Protein vaccines IIIa: Protecting against a deleterious foreign agent IIIb: Treating an autoimmune disease IIIc: Treating cancer
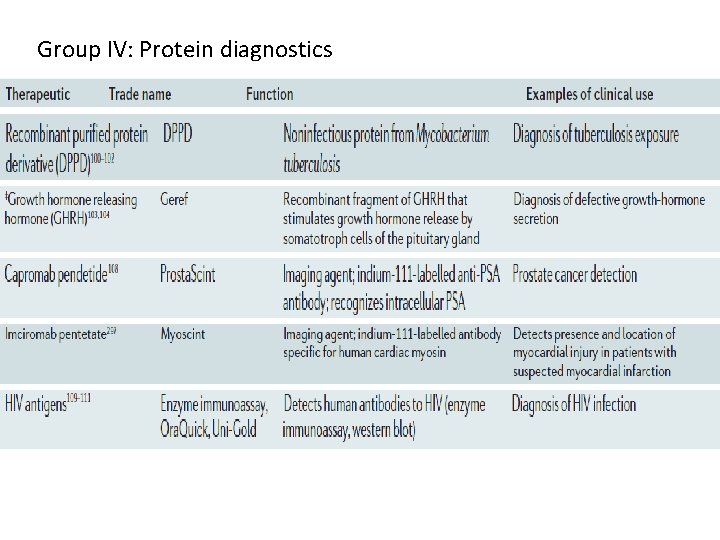
Group IV: Protein diagnostics

Challenges for protein therapeutics: 1. Protein solubility, route of administration, distribution, and stability. 2. Immune response. 3. Physiologically active – post-translational modifications. 4. Costs 5. Ethics

Recombinant human proteins: -FDA – biotechnology medicines: -Monoclonal antibodies -Natural interferons -Vaccines -Hormones -Modified natural enzymes -Cell therapies More work needs to be done.
- Slides: 10