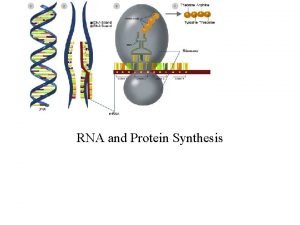
Protein Synthesis TRANSLATION Why is protein very important


















- Slides: 156

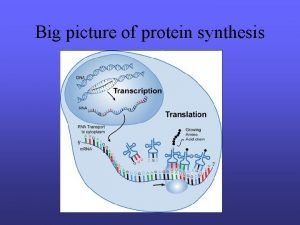
Protein Synthesis (TRANSLATION)

Why is protein very important? It is the essential structural part of every cell n It performs multiple important roles in all biological processes. n No life without protein How they are synthesised? ? ?

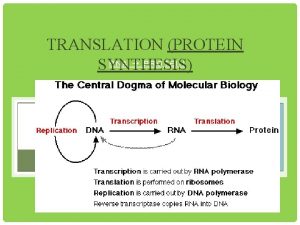
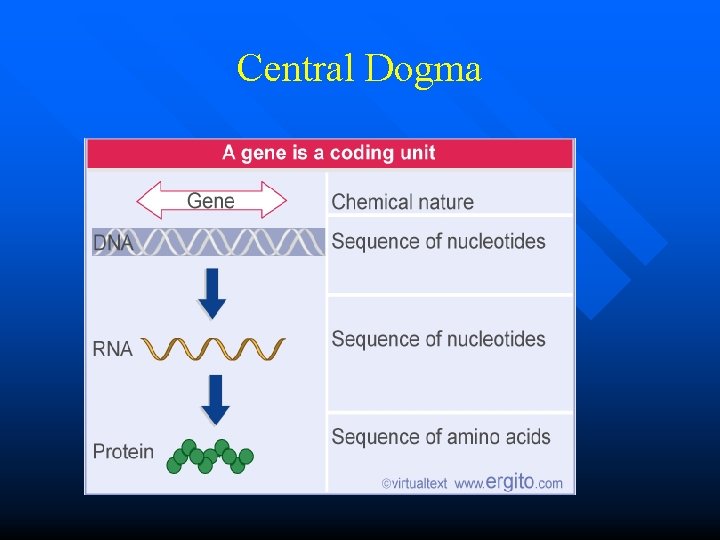
Central Dogma

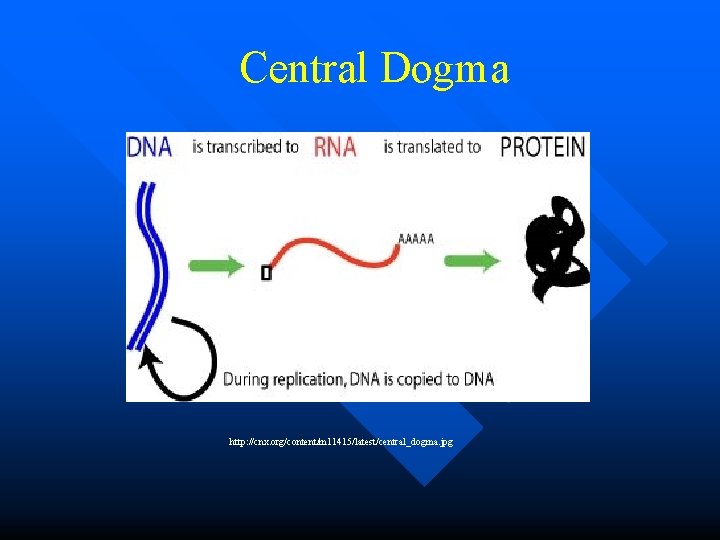
Central Dogma http: //cnx. org/content/m 11415/latest/central_dogma. jpg

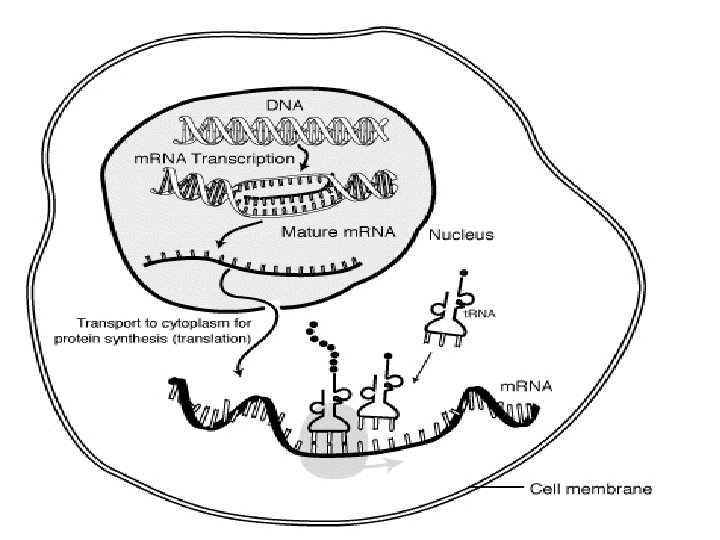
n The production of a protein molecule by a cell is the result of a two part process. Transcription Translation

The transfer of genetic information from DNA to RNA is known as transcription.

Transcription n occurs in the nucleus of the eukaryotic cell 1) DNA strand separates and serves as a template (pattern) for m. RNA assembly

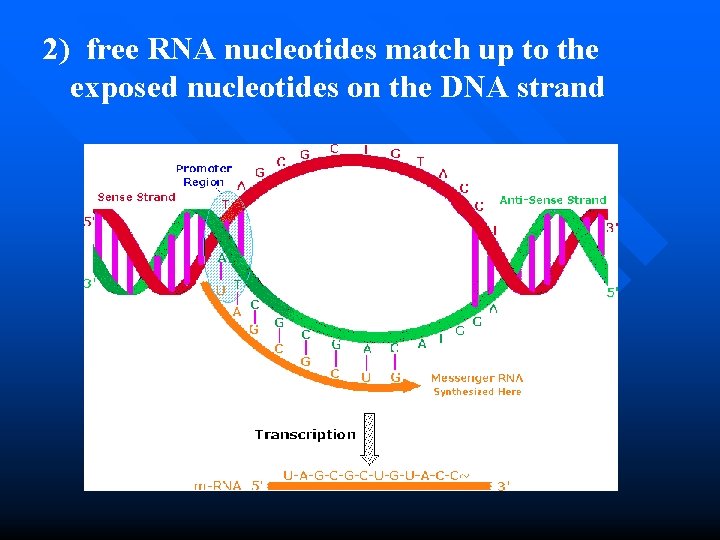
2) free RNA nucleotides match up to the exposed nucleotides on the DNA strand

3) m. RNA strand leaves the DNA strand when a “stop signal” is reached 4) the m. RNA strand carries the code for the production of one polypeptide

The second part of the process, called translation, occurs in the cytoplasm and results in the synthesis of a specific protein molecule from the genetic information carried by the m. RNA.

Translation occurs in the cytoplasm of the eukaryotic cell 1) m. RNA moves out of the nucleus and into the cytoplasm to a ribosome n 2) m. RNA is “read” by the ribosome and is converted to a chain of amino acids with the help of t. RNA

Translation 3) As the m. RNA moves across the ribosome, t. RNAs temporarily attach. The amino acids are joined by a chemical bond by enzymes until a stop codon is reached 4) a polypeptide is produced


DNA is transcribed to m. RNA in the nucleus and m. RNA is translated into Protein in the cytoplasm

TRANSLATION n The m. RNA are short lived and immediately translated. n In translation process proteins are synthesized.

Protein Synthesis Material RNA (m. RNA, t. RNAs, r. RNAs) n Amino acids (20 common AA) n Ribosomes (r. RNA+proteins) n Enzymes and protein factors n Cations ( Mg 2+, K+) n Energy (ATP, GTP) n

RNA n RNA, like DNA, is a polymer formed by a sequence of nucleotides n Three main types of RNA: messenger RNA (m. RNA)---template transfer RNA (t. RNA)--- transfer amino acids ribosomal RNA (r. RNA)---form ribosome with proteins, which provides place for translation

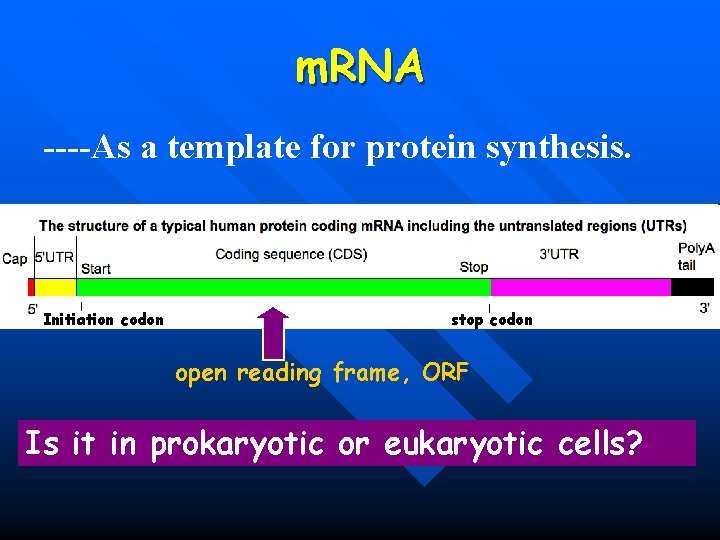
m. RNA ----As a template for protein synthesis. Initiation codon stop codon open reading frame, ORF Is it in prokaryotic or eukaryotic cells?

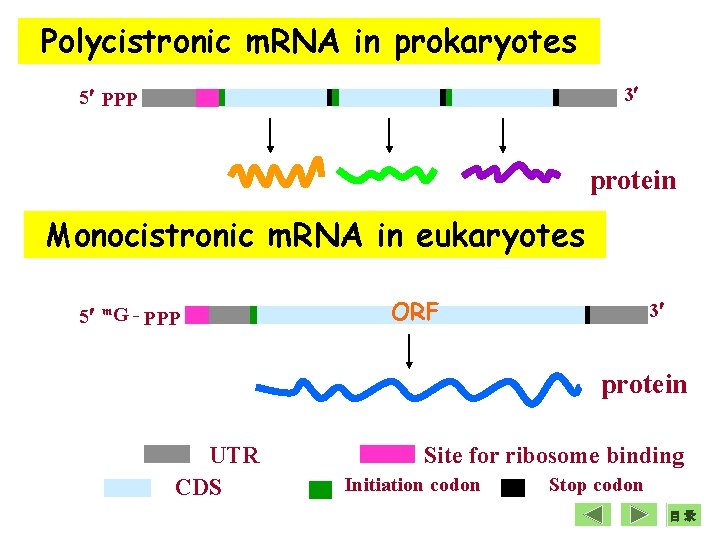
Polycistronic m. RNA in prokaryotes 3 5 PPP protein Monocistronic m. RNA in eukaryotes 5 m. G - PPP ORF 3 protein UTR CDS Site for ribosome binding Initiation codon Stop codon 目录

4 nucleotides in m. RNA 20 amino acids in protein 目录

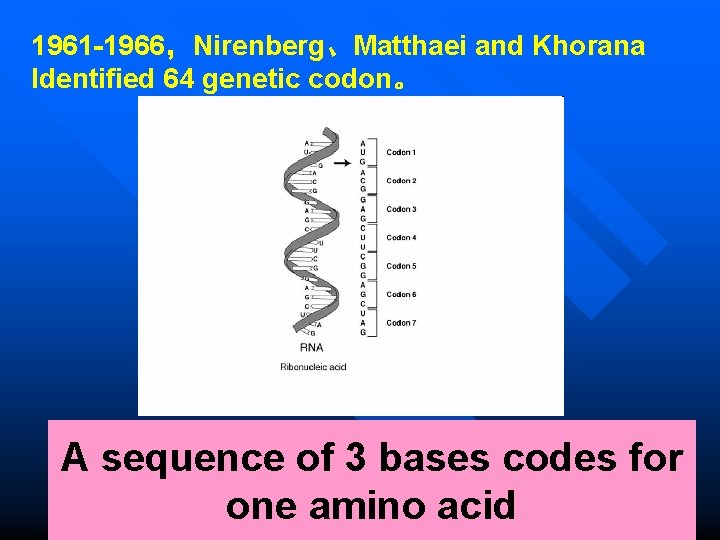
1961 -1966,Nirenberg、Matthaei and Khorana Identified 64 genetic codon。 A sequence of 3 bases codes for one amino acid

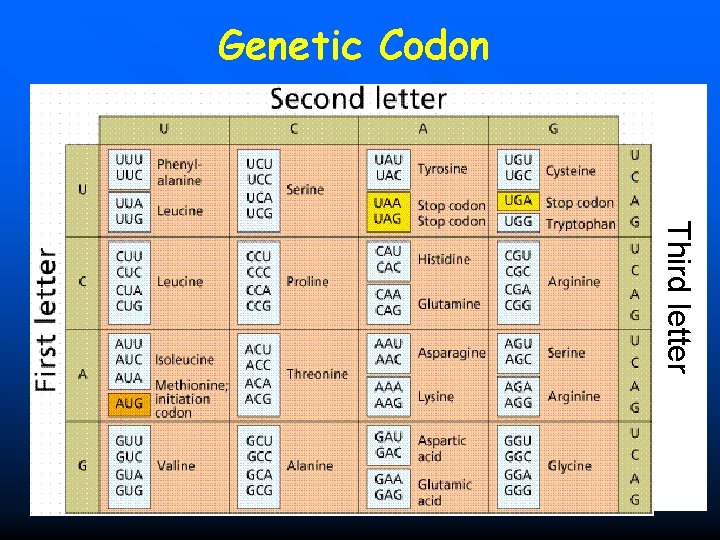
n Genetic Codon (Triplet codon) It is a triplet code consisting of sequence of three nucleotides, which codes for a specific amino acid, or stands for initiation and stop signals. 149

n 64 genetic codons n Initiation codon: AUG (Methionine) n Stop codon: UAA UAG UGA n 61 codons for 20 amino acids

Genetic Codon Third letter

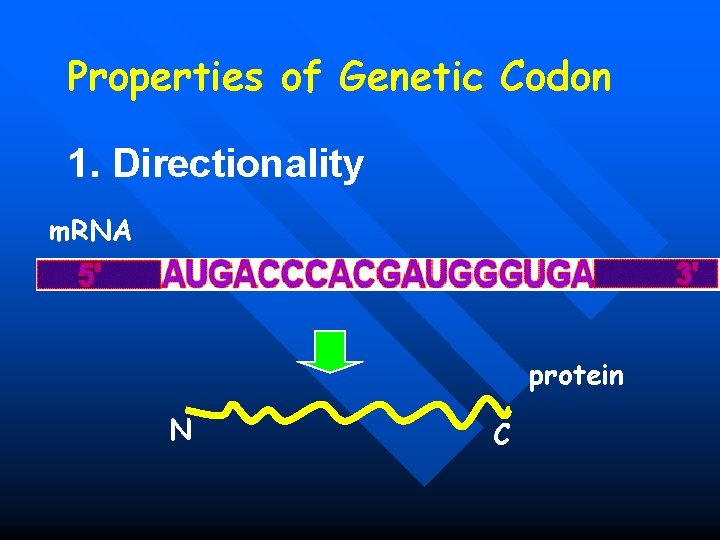
Properties of Genetic Codon 1. Directionality m. RNA protein N C

Properties of Genetic Codon 2. Commaless (Non-punctuation and nonoverlapping) m. RNA successively

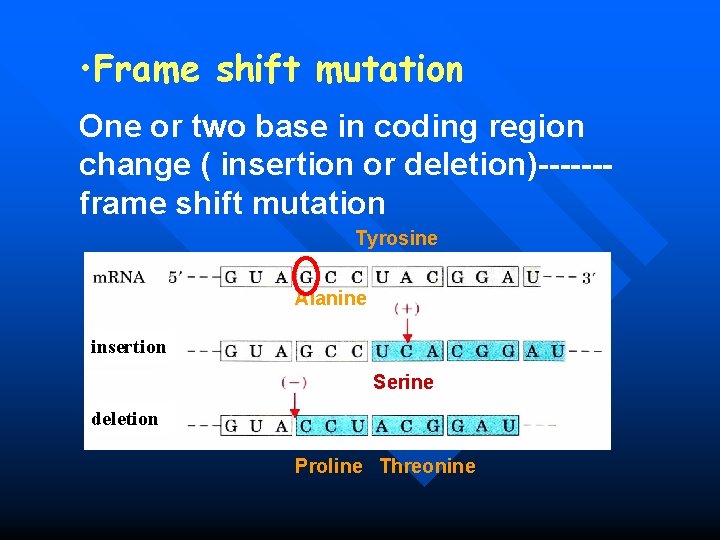
• Frame shift mutation One or two base in coding region change ( insertion or deletion)------frame shift mutation Tyrosine Alanine insertion Serine deletion Proline Threonine

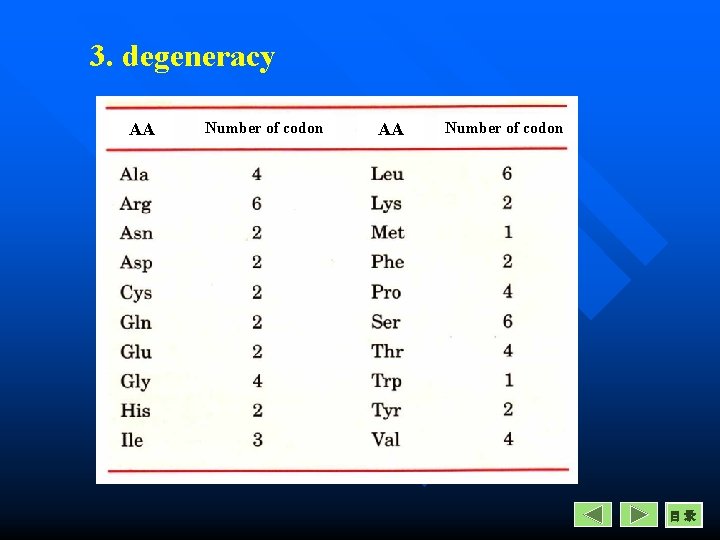
Properties of Genetic Codon 3. Degeneracy Except Methionine and Tryptophan, every amino acid is encoded by more than one codon. 149

3. degeneracy AA Number of codon 目录

3. Degeneracy Gly GGU GGC GGA GGG 目录

3. Degeneracy In general, the specificity of codon is determined by the first two letters, since third letter is less important. If the third letter of genetic codon changes, it might not change the amino acid and affect the stucture of the synthesized protein.

Properties of Genetic Codon 4. Universality The genetic codons are the same in almost all organisms with some rare exception( human mitochondrial m. RNA) The universality indicates that all living things share a common evolutionary heritage.

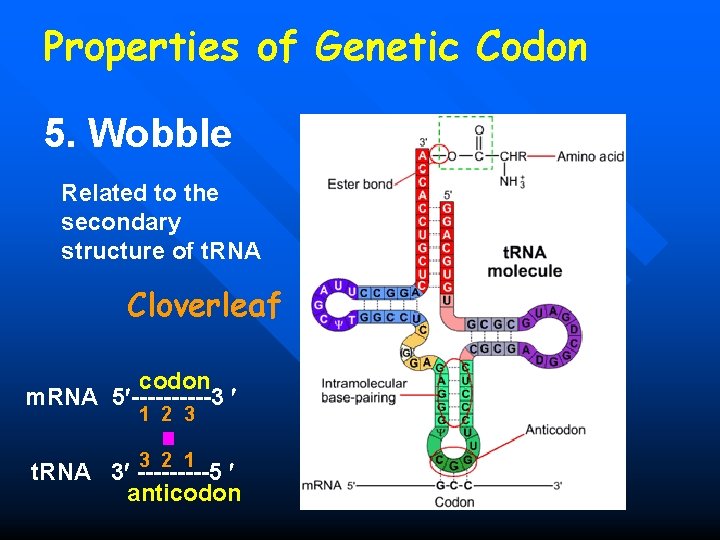
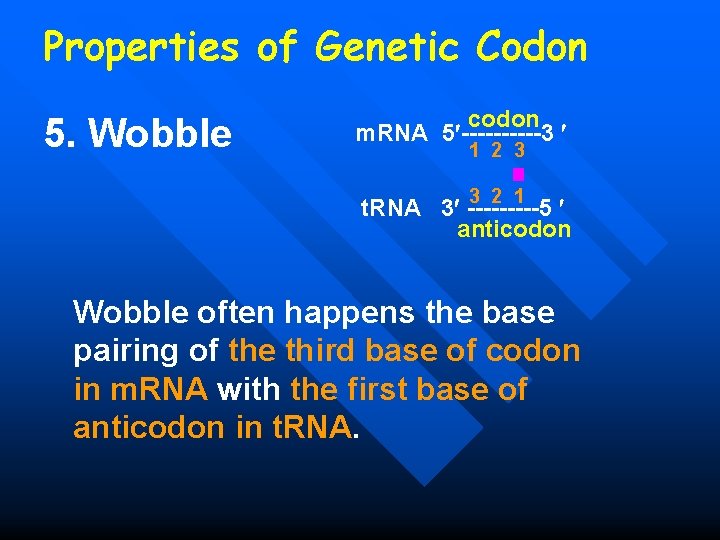
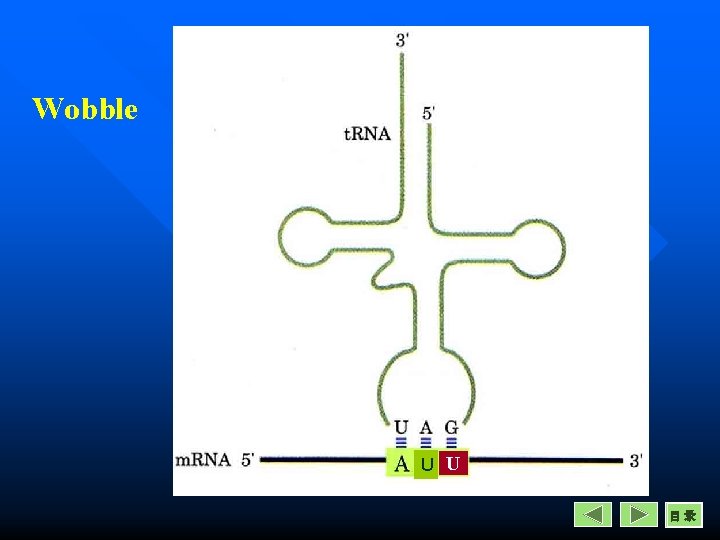
Properties of Genetic Codon 5. Wobble Related to the secondary structure of t. RNA Cloverleaf codon m. RNA 5 -----3 1 2 3 3 2 1 t. RNA 3 -----5 anticodon

Properties of Genetic Codon 5. Wobble codon m. RNA 5 -----3 1 2 3 3 2 1 t. RNA 3 -----5 anticodon Wobble often happens the base pairing of the third base of codon in m. RNA with the first base of anticodon in t. RNA.

Wobble U U 目录

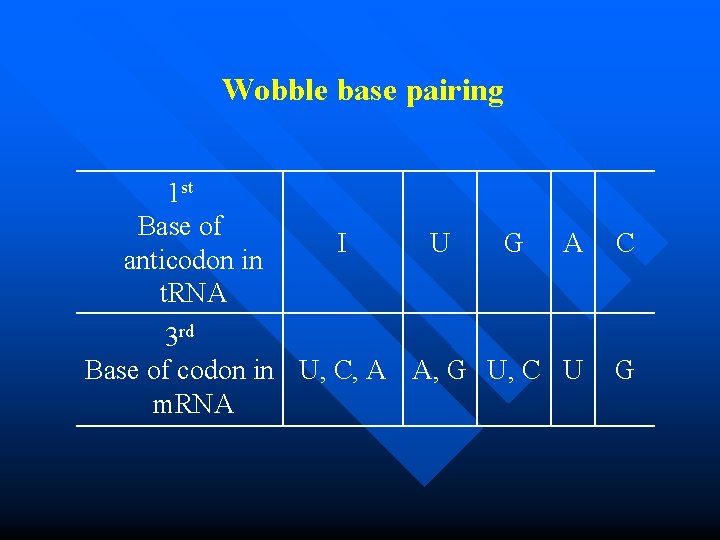
Wobble base pairing 1 st Base of anticodon in t. RNA I U G A C 3 rd Base of codon in U, C, A A, G U, C U m. RNA G

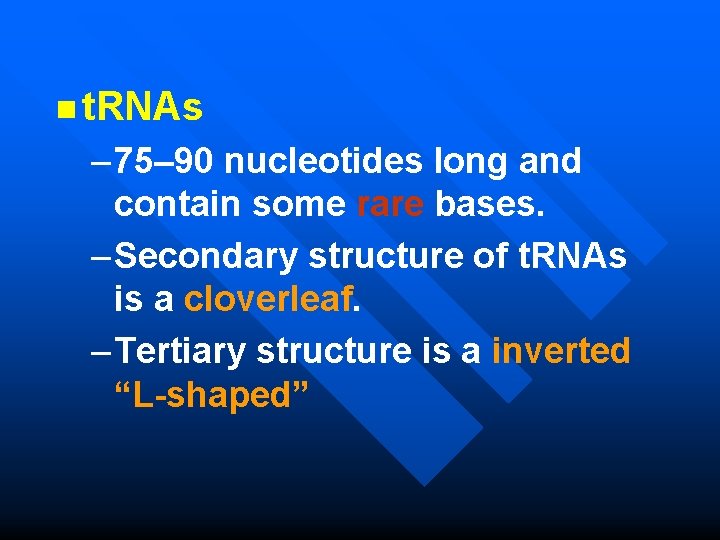
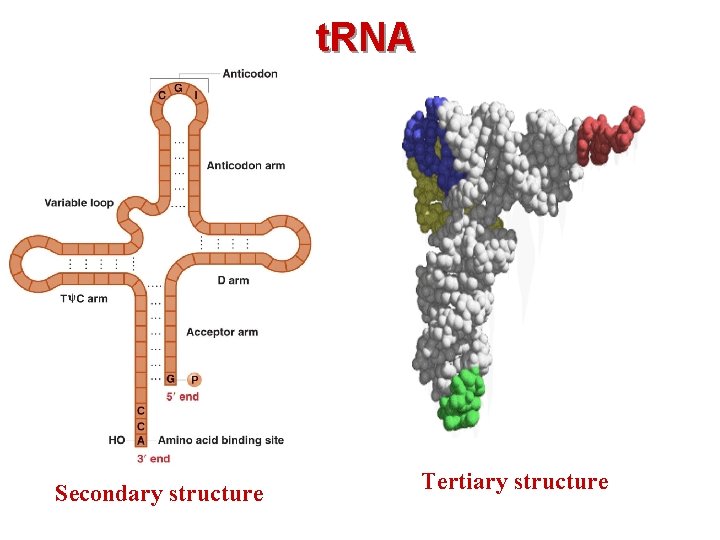
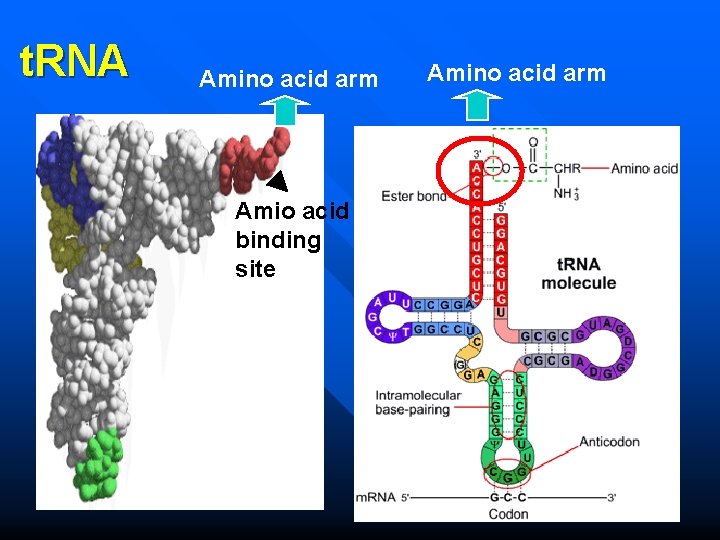
n t. RNAs – 75– 90 nucleotides long and contain some rare bases. – Secondary structure of t. RNAs is a cloverleaf. – Tertiary structure is a inverted “L-shaped”

t. RNA Secondary structure Tertiary structure

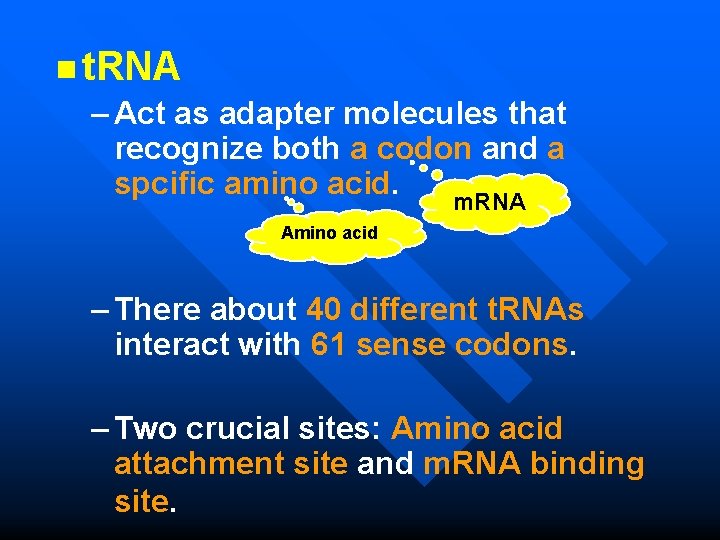
n t. RNA – Act as adapter molecules that recognize both a codon and a spcific amino acid. m. RNA Amino acid – There about 40 different t. RNAs interact with 61 sense codons. – Two crucial sites: Amino acid attachment site and m. RNA binding site.

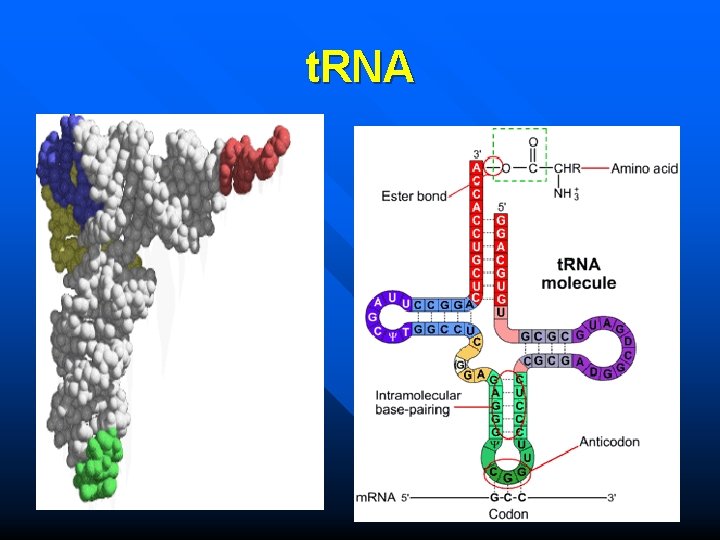
t. RNA

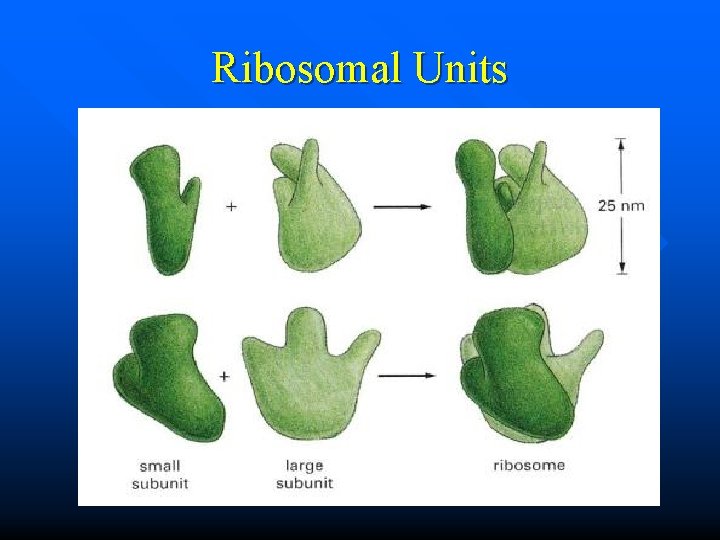
n Ribosomes – consist of ribosomal proteins and ribosomal RNAs (r. RNAs). – have a large subunit and a small subunit. http: //image. wistatutor. com/content/feed/u 2044/ribosomes%20%20 function. jpg

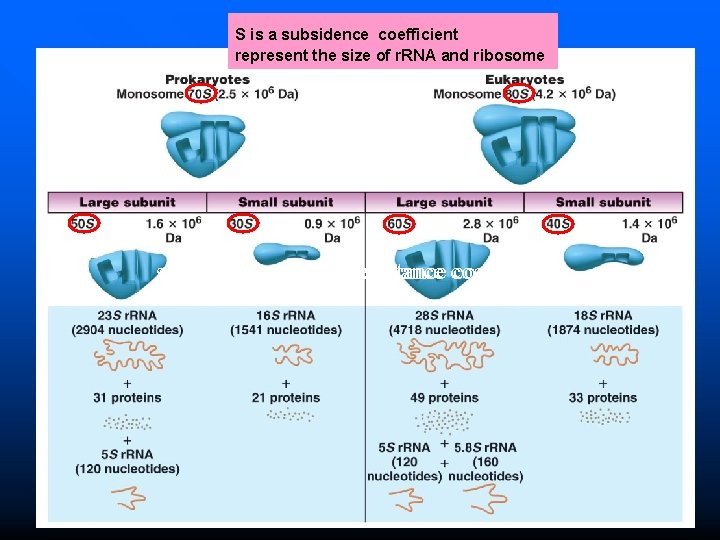
Ribosomal Units

S is a subsidence coefficient represent the size of r. RNA and ribosome subsidence rate and resistance coefficient Figure 15. 1

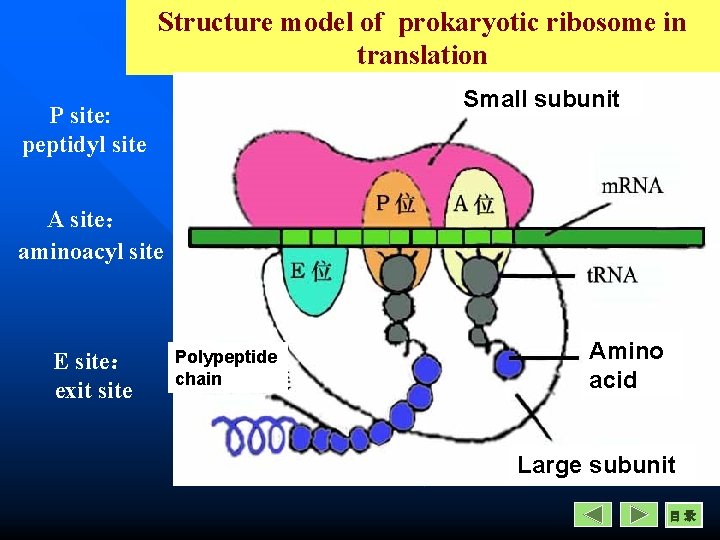
n Ribosomes – Small subunit can bind initiator t. RNA and m. RNA for initiaion. – There are two binding sites: A site (binding incoming aminoacyl-t. RNA) P site( binding the growing polypeptide chain) E site (only in prokaryotic ribosome, empty t. RNA exit from this site)

Structure model of prokaryotic ribosome in translation Small subunit P site: peptidyl site A site: aminoacyl site E site: exit site Polypeptide chain Amino acid Large subunit 目录

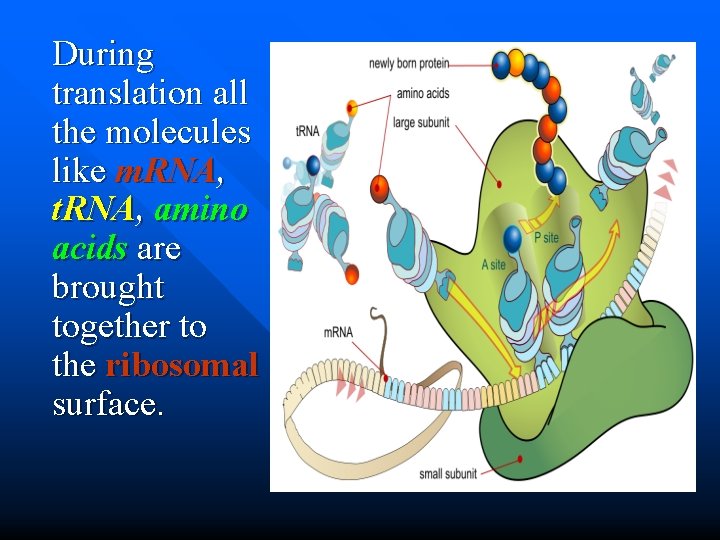
During translation all the molecules like m. RNA, t. RNA, amino acids are brought together to the ribosomal surface.

Studies for the structure and function of the ribosome ------The Nobel Prize in Chemistry, 2009 Venkatraman Ramakrishnan Thomas A. Steitz Ada E. Yonath

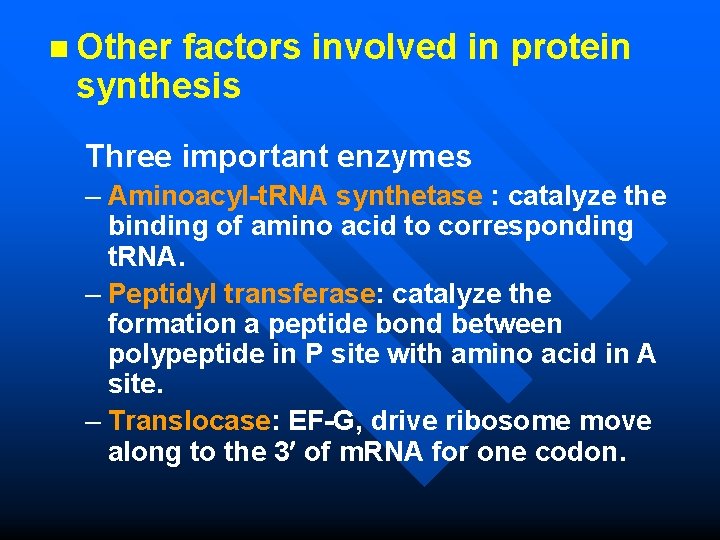
n Other factors involved in protein synthesis Three important enzymes – Aminoacyl-t. RNA synthetase : catalyze the binding of amino acid to corresponding t. RNA. – Peptidyl transferase: catalyze the formation a peptide bond between polypeptide in P site with amino acid in A site. – Translocase: EF-G, drive ribosome move along to the 3 of m. RNA for one codon.

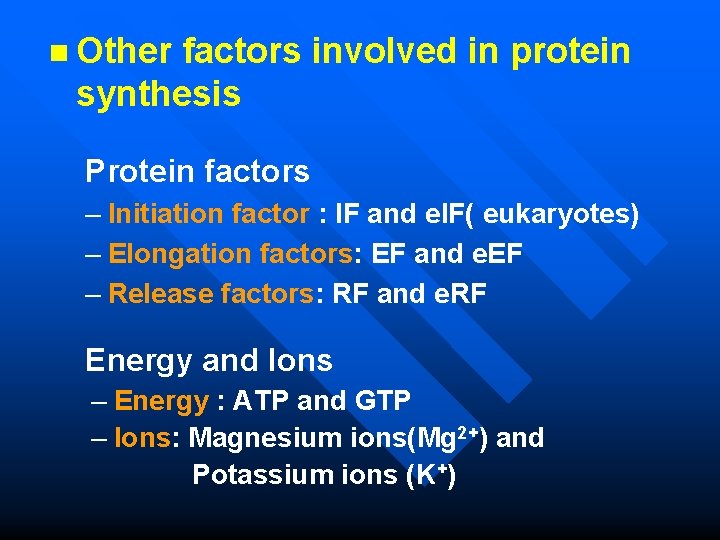
n Other factors involved in protein synthesis Protein factors – Initiation factor : IF and e. IF( eukaryotes) – Elongation factors: EF and e. EF – Release factors: RF and e. RF Energy and Ions – Energy : ATP and GTP – Ions: Magnesium ions(Mg 2+) and Potassium ions (K+)

Amino acid activation n Amino acid are present freely in the cytoplasm in the inactive state. n Amino acid activation means the amino acid is attached to a corresponding t. RNA. 149

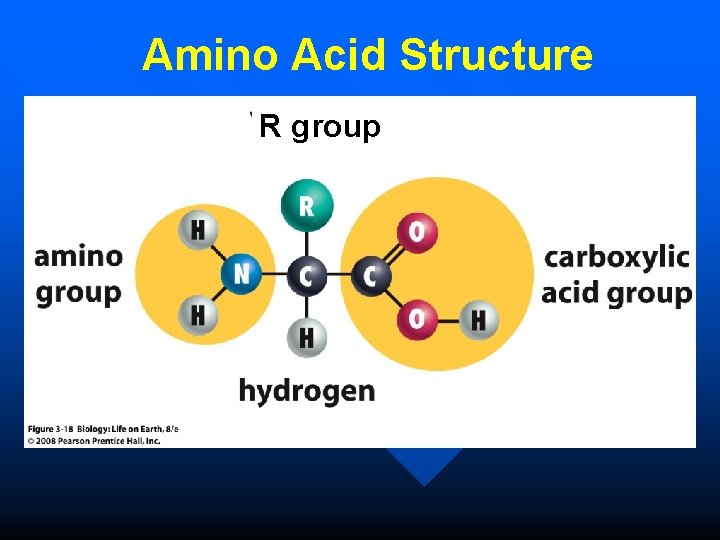
n all amino acids have: – a central carbon atom – a carboxylic group – an amino group – a hydrogen atom – an R (radical) group n The R group of an amino acid confers specific chemical properties.

Amino Acid Structure R group

t. RNA Amino acid arm Amio acid binding site Amino acid arm

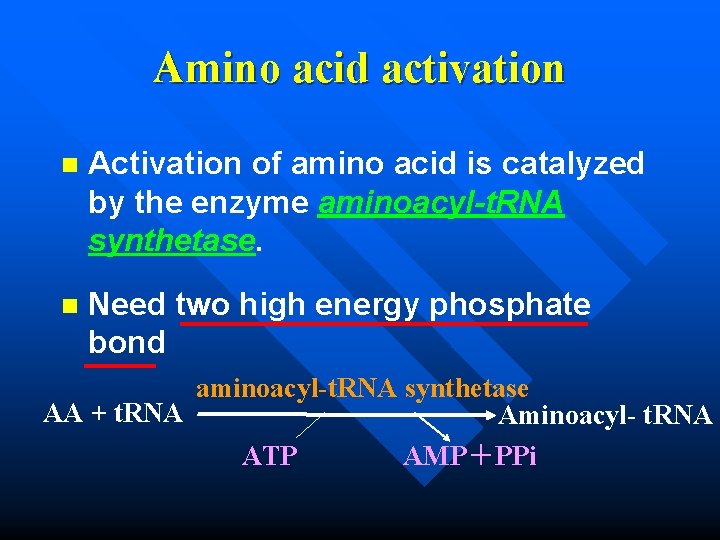
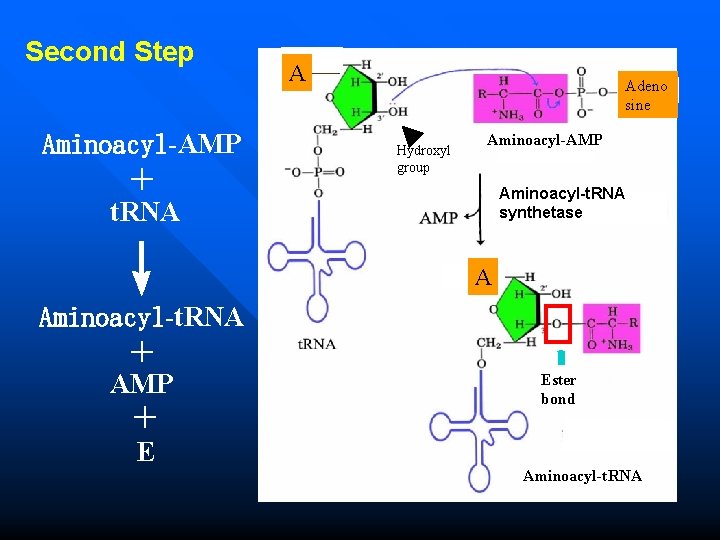
Amino acid activation n Activation of amino acid is catalyzed by the enzyme aminoacyl-t. RNA synthetase. n Need two high energy phosphate bond aminoacyl-t. RNA synthetase AA + t. RNA Aminoacyl- t. RNA ATP AMP+PPi

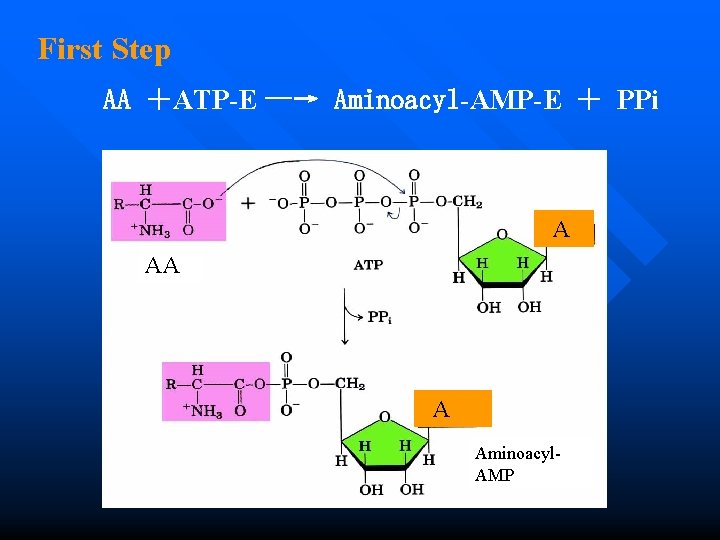
Amino acid activation n First Step During the first step of amino acid activation, ATP breaks into AMP which forms complex with amino acid and pyrophosphate.

First Step AA +ATP-E —→ Aminoacyl-AMP-E + PPi A AA A Aminoacyl. AMP

Second Step A Adeno sine Aminoacyl-AMP + t. RNA Hydroxyl group Aminoacyl-t. RNA synthetase ↓ Aminoacyl-t. RNA + AMP + E Aminoacyl-AMP A A 酯键 Ester bond Aminoacyl-t. RNA

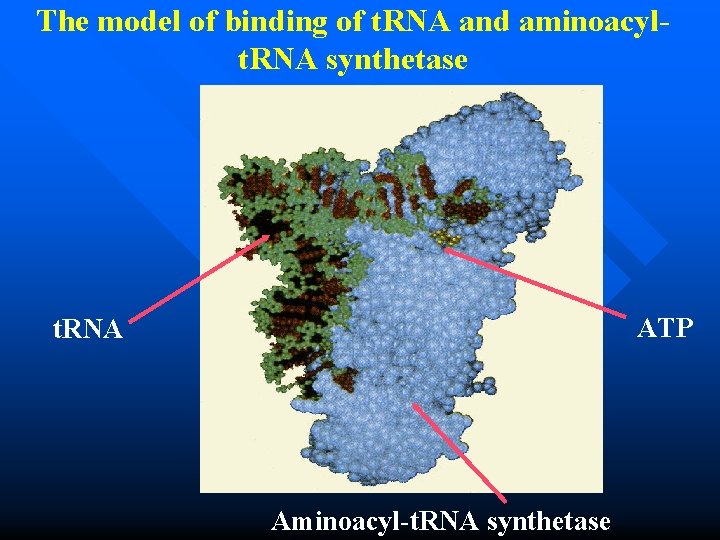
The model of binding of t. RNA and aminoacylt. RNA synthetase ATP t. RNA Aminoacyl-t. RNA synthetase

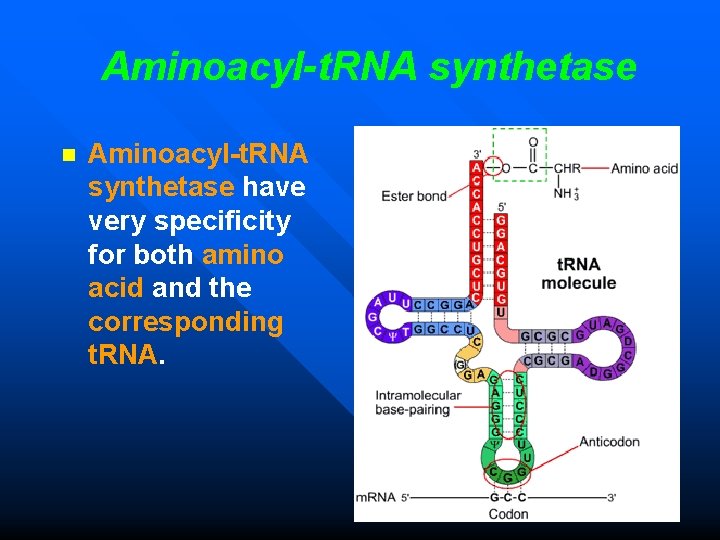
Aminoacyl-t. RNA synthetase n Aminoacyl-t. RNA synthetase have very specificity for both amino acid and the corresponding t. RNA.

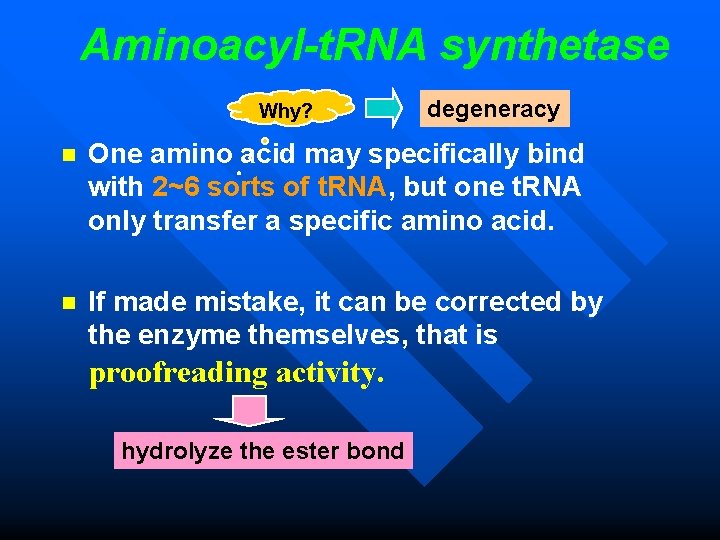
Aminoacyl-t. RNA synthetase Why? degeneracy n One amino acid may specifically bind with 2~6 sorts of t. RNA, but one t. RNA only transfer a specific amino acid. n If made mistake, it can be corrected by the enzyme themselves, that is proofreading activity. hydrolyze the ester bond

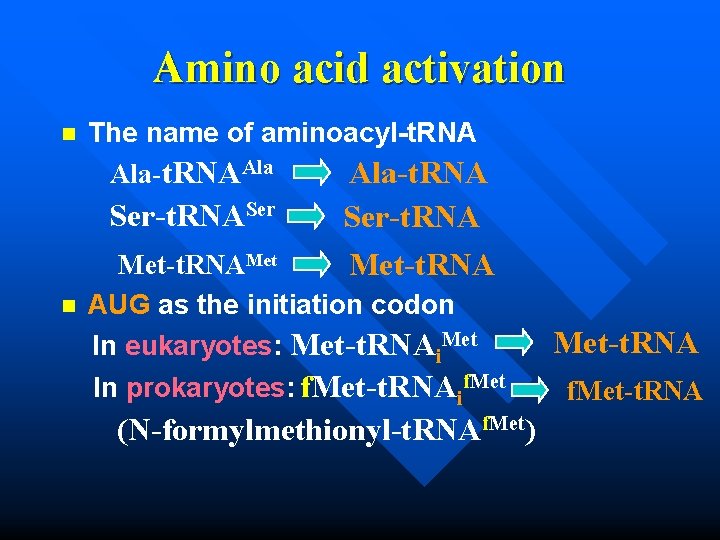
Amino acid activation n The name of aminoacyl-t. RNA Ala-t. RNAAla Ser-t. RNASer Ser-t. RNA Met-t. RNAMet n AUG as the initiation codon In eukaryotes: Met-t. RNAi. Met In prokaryotes: f. Met-t. RNAif. Met (N-formylmethionyl-t. RNAf. Met) Met-t. RNA f. Met-t. RNA

Summary for Amino acid activation n The activated amino acid is attached to specific t. RNA by specific enzyme aminoacyl-t. RNA synthetase. n The amino acid gets attached to the 3 terminal of –CCA in t. RNA, forming an ester bond. n Each enzyme recognizes a single amino acid and correct t. RNA to which it is to be attached. If made mistake, it has proofreading activity.

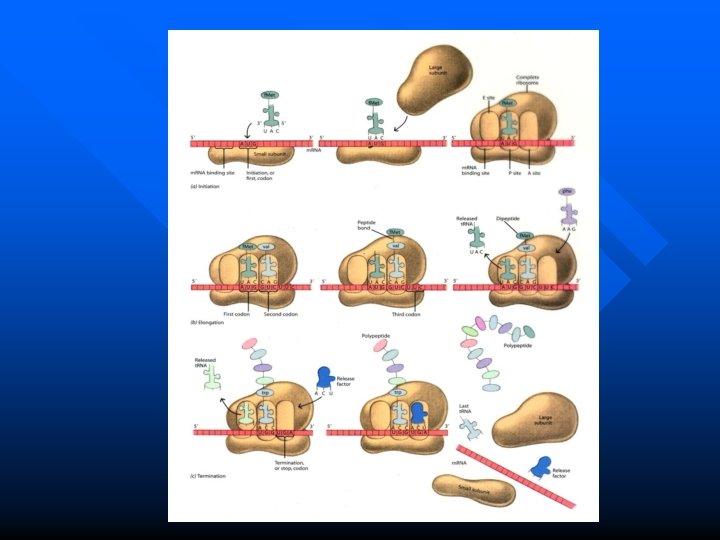
The Process of Translation involes three steps n Initiation n Elongation n Termination

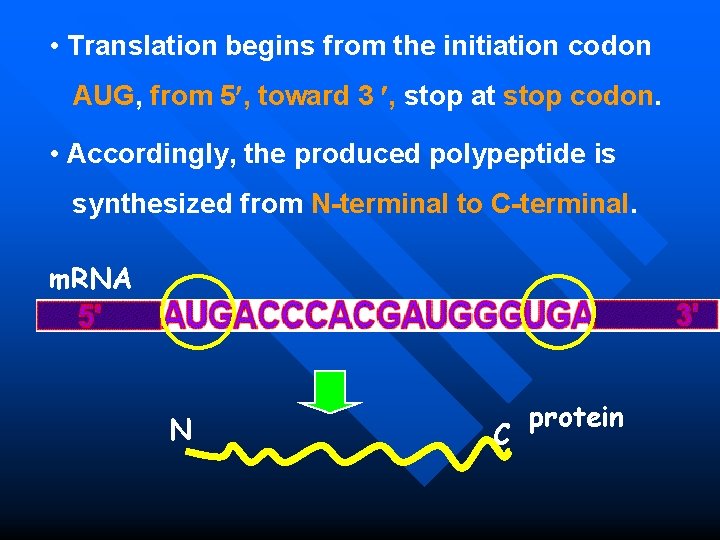
• Translation begins from the initiation codon AUG, from 5 , toward 3 , stop at stop codon. • Accordingly, the produced polypeptide is synthesized from N-terminal to C-terminal. m. RNA N C protein

Initiation of polypeptide chain The actual sites of protein synthesis are ribosomes. n The ribosomes are composed of two subunits – 30 S, 50 S in prokaryotes, 40 S, 60 S in eukaryotes. n There are two sites in ribosomes: A site and P site (E site in prokaryotes) n

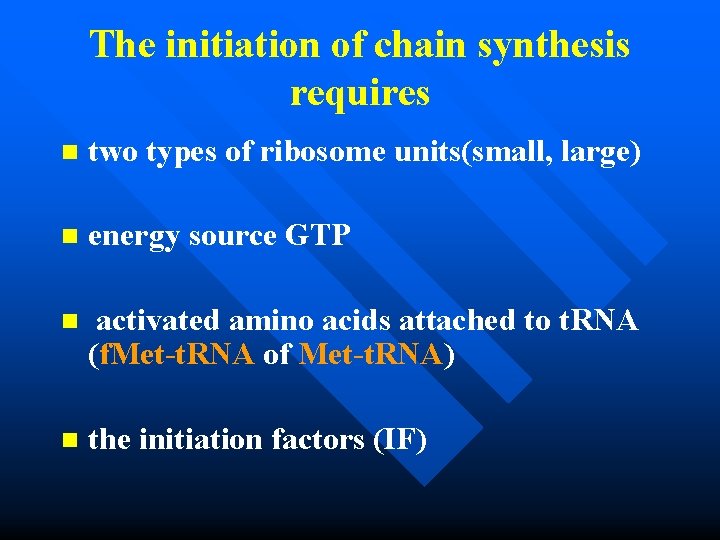
The initiation of chain synthesis requires n two types of ribosome units(small, large) n energy source GTP n activated amino acids attached to t. RNA (f. Met-t. RNA of Met-t. RNA) n the initiation factors (IF)

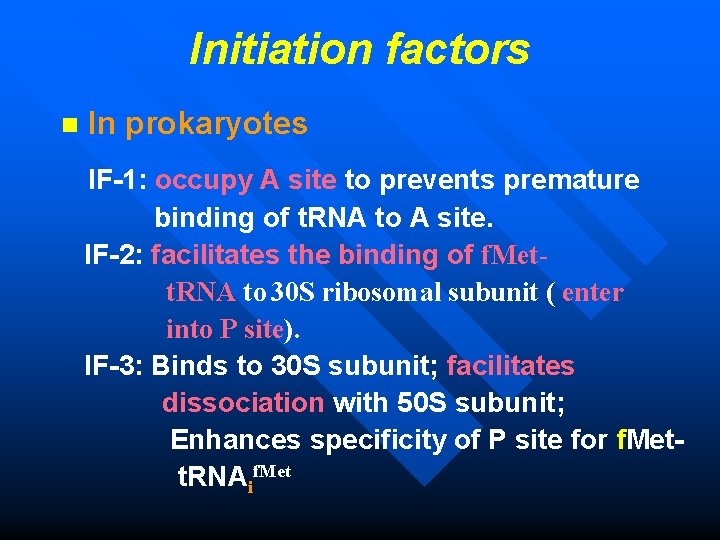
Initiation factors n In prokaryotes IF-1: occupy A site to prevents premature binding of t. RNA to A site. IF-2: facilitates the binding of f. Mett. RNA to 30 S ribosomal subunit ( enter into P site). IF-3: Binds to 30 S subunit; facilitates dissociation with 50 S subunit; Enhances specificity of P site for f. Met t. RNAif. Met

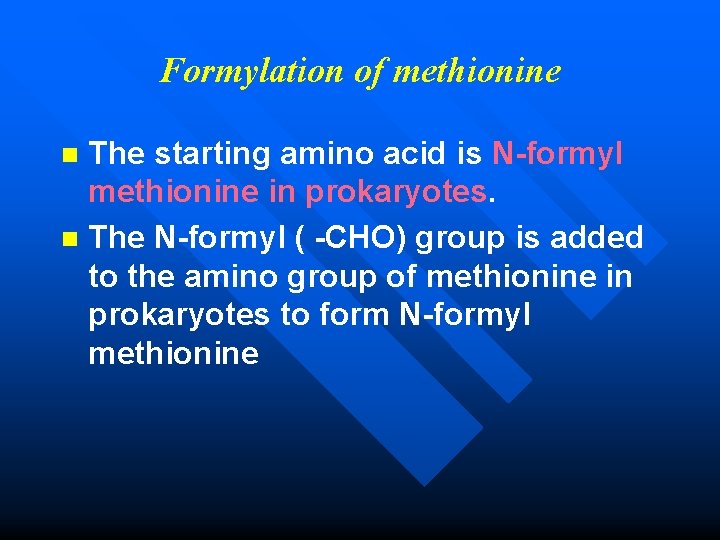
Formylation of methionine The starting amino acid is N-formyl methionine in prokaryotes. n The N-formyl ( -CHO) group is added to the amino group of methionine in prokaryotes to form N-formyl methionine n

Initiation n Refers to the process of m. RNA and f. Met-t. RNA binding with ribosome respectively, to form translational initiation complex.

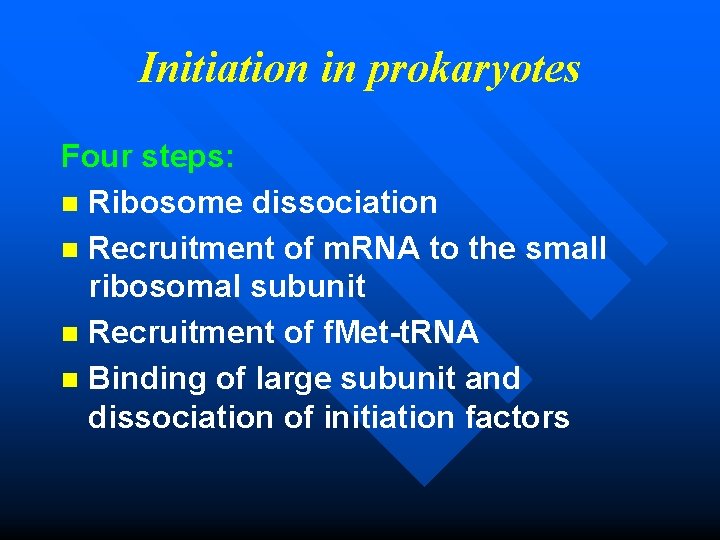
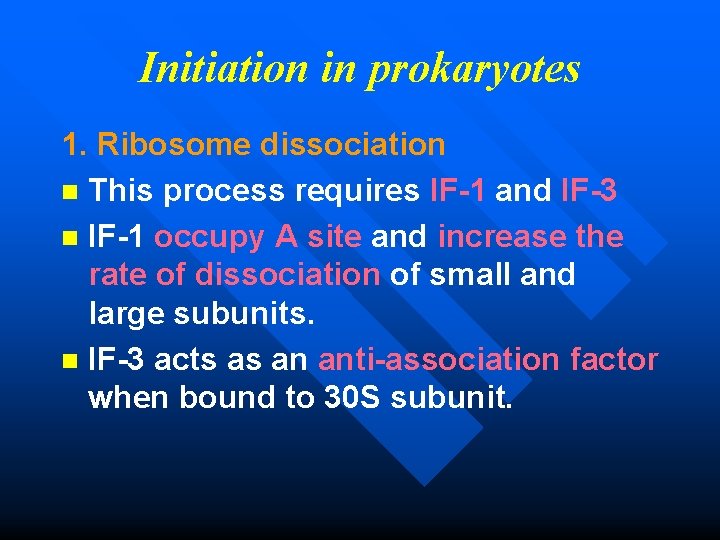
Initiation in prokaryotes Four steps: n Ribosome dissociation n Recruitment of m. RNA to the small ribosomal subunit n Recruitment of f. Met-t. RNA n Binding of large subunit and dissociation of initiation factors

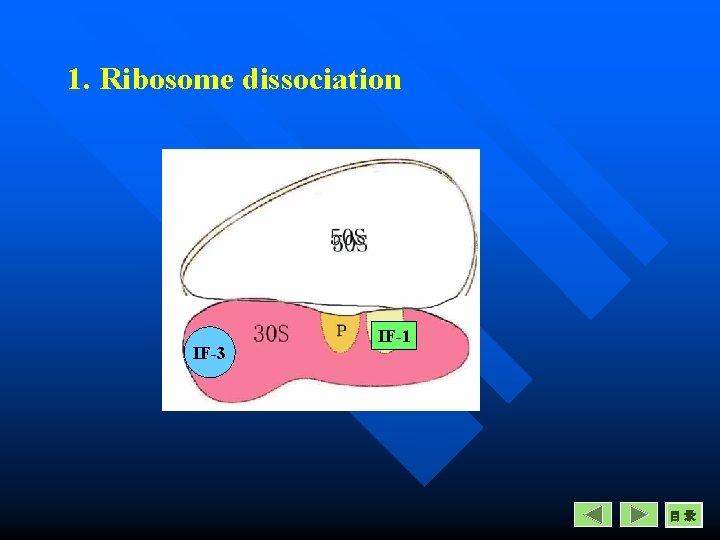
Initiation in prokaryotes 1. Ribosome dissociation n This process requires IF-1 and IF-3 n IF-1 occupy A site and increase the rate of dissociation of small and large subunits. n IF-3 acts as an anti-association factor when bound to 30 S subunit.

1. Ribosome dissociation IF-3 IF-1 目录

Initiation in prokaryotes 2. Recruitment of m. RNA to the small ribosomal subunit AUG is correctly positioned to P site. n How can m. RNA precisely bind to the small ribosomal subunit ? n Depends on recognition between small subunit and m. RNA n

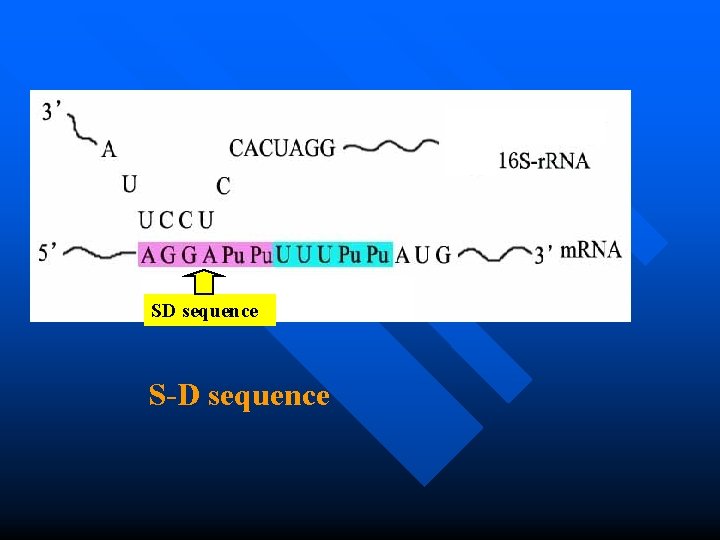
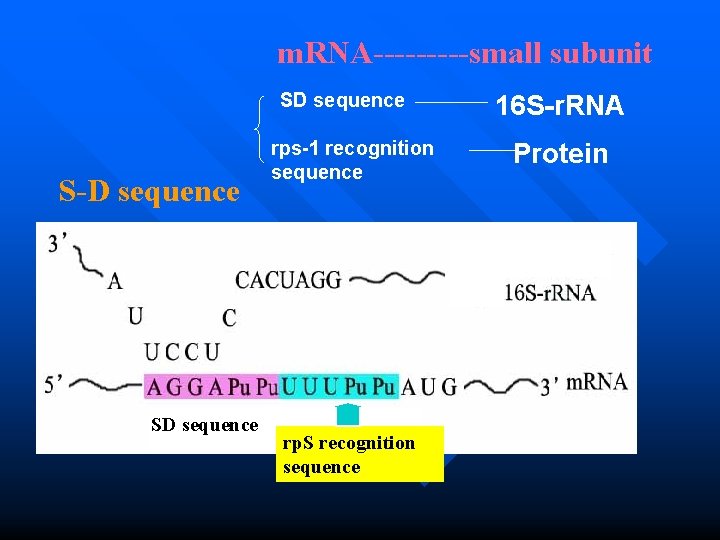
Initiation in prokaryotes How do m. RNA and small subunit recognize each other? n which is assisted by base pairing between the conserved sequence UCCU at 3 of 16 S r. RNA and the SD sequence AGGA at 5 of m. RNA n SD sequence: which is an initiation signal of 4~9 purine residues, 8~13 bp upstream to the AUG,only in prokaryotic m. RNA. 149 n This is a RNA-RNA recognition

Small subunit SD sequence S-D sequence

Initiation in prokaryotes 2. Recruitment of m. RNA to the small ribosomal subunit n There is also a RNA-protein recognition between m. RNA and rp. S. n rp. S recognition sequence: between SD sequence and AUG, which can couple with small ribosomal protein(rp. S)

m. RNA-----small subunit SD sequence S-D sequence rps-1 recognition sequence 16 S-r. RNA Protein Small subunit SD sequence rp. S recognition sequence

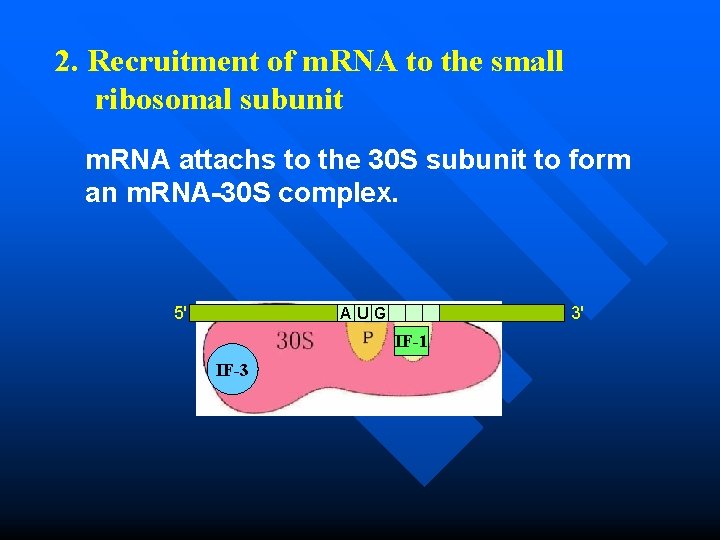
2. Recruitment of m. RNA to the small ribosomal subunit m. RNA attachs to the 30 S subunit to form an m. RNA-30 S complex. 5' 3' AUG IF-1 IF-3

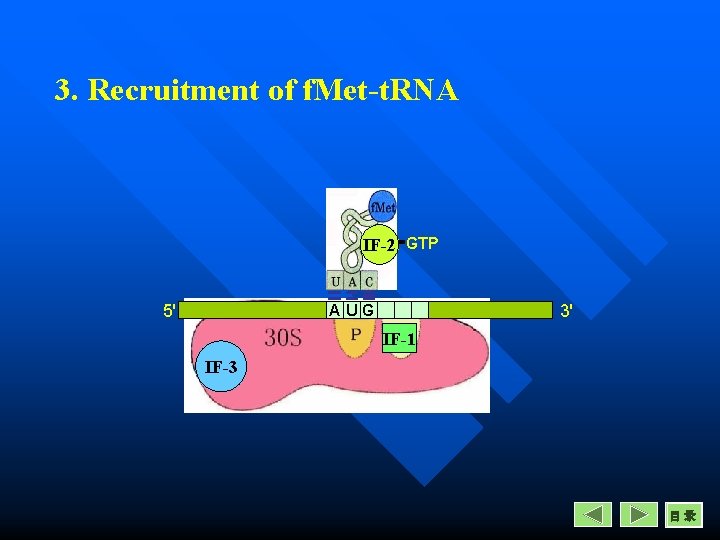
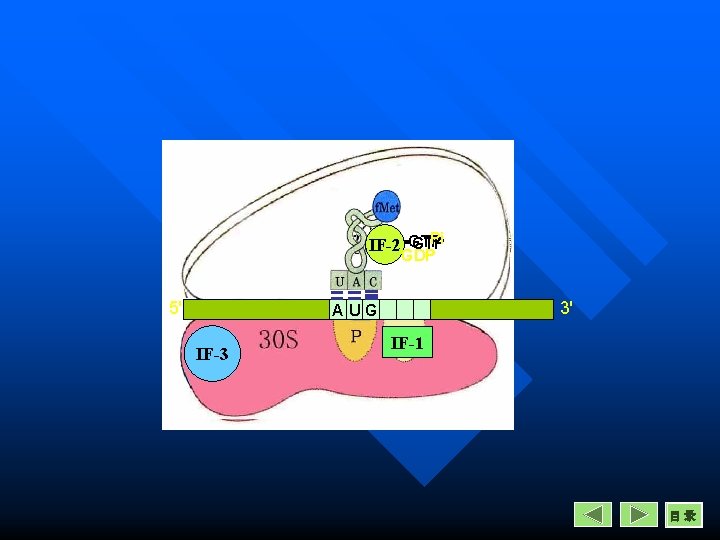
Initiation in prokaryotes 3. Recruitment of f. Met-t. RNA n n A site is occupyed by IF-1 The f. Met-t. RNA comes to P site with the help of IF-2 and GTP. The f. Met-t. RNA is attached by its anticodon CAU to the initiation codon AUG in m. RNA. The f. Met-t. RNA complex binds with the m. RNA-30 S complex to form the completed 30 S initiation complex.

3. Recruitment of f. Met-t. RNA IF-2 GTP 5' 3' AUG IF-1 IF-3 目录

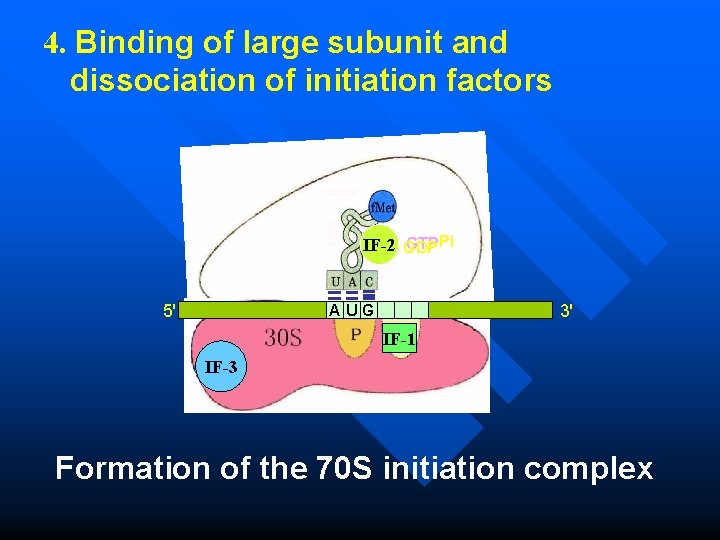
Initiation in prokaryotes 4. Binding of large subunit and dissociation of initiation factors n n n GTP hydrolysis facilitates the binding of 50 S subunit to 30 S subunit. The initiation factors are released. Form the 70 S initiation complex. The m. RNA now positioned in a groove between two ribosomal subunits. Now, P site is occupied by f. Met-t. RNA, A site is empty for a new aminoacyl-t. RNA to enter.

4. Binding of large subunit and dissociation of initiation factors GTPPi IF-2 GDP 5' 3' AUG IF-1 IF-3 Formation of the 70 S initiation complex

Pi GTP IF-2 -GTP GDP 5' 3' AUG IF-3 IF-1 目录

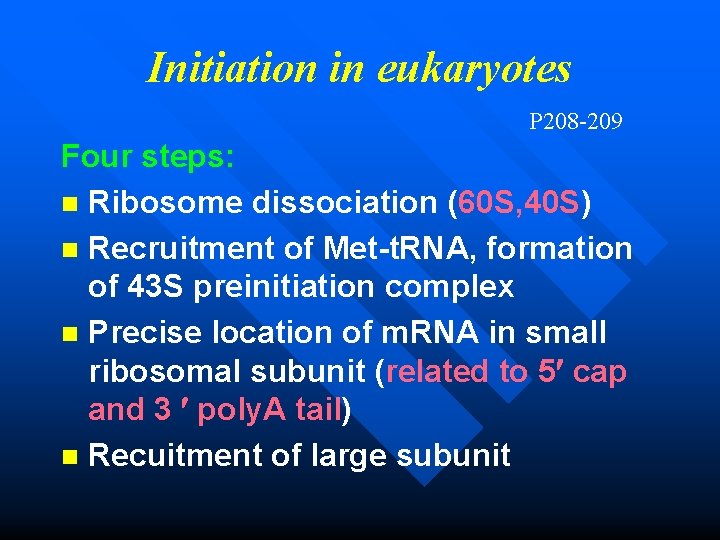
Initiation in eukaryotes P 208 -209 Four steps: n Ribosome dissociation (60 S, 40 S) n Recruitment of Met-t. RNA, formation of 43 S preinitiation complex n Precise location of m. RNA in small ribosomal subunit (related to 5 cap and 3 poly. A tail) n Recuitment of large subunit

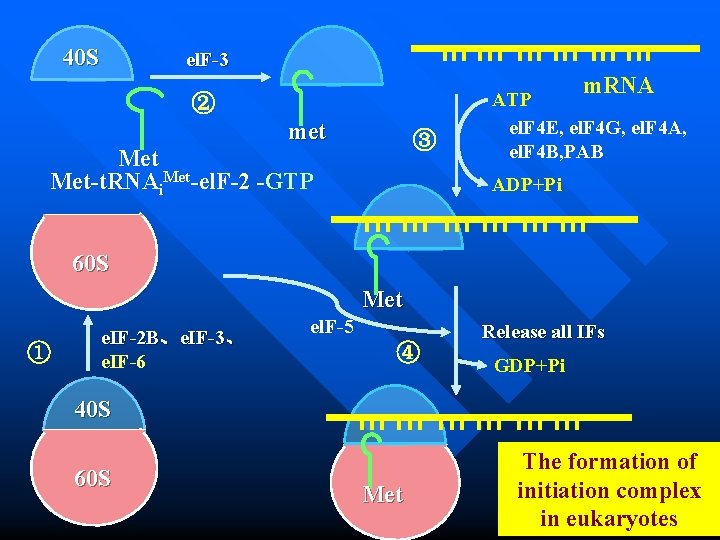
40 S el. F-3 m. RNA ② met ③ Met-t. RNAi. Met-el. F-2 -GTP ATP el. F 4 E, el. F 4 G, el. F 4 A, el. F 4 B, PAB ADP+Pi 60 S Met ① e. IF-2 B、e. IF-3、 e. IF-6 el. F-5 ④ Release all IFs GDP+Pi 40 S 60 S Met The formation of initiation complex in eukaryotes

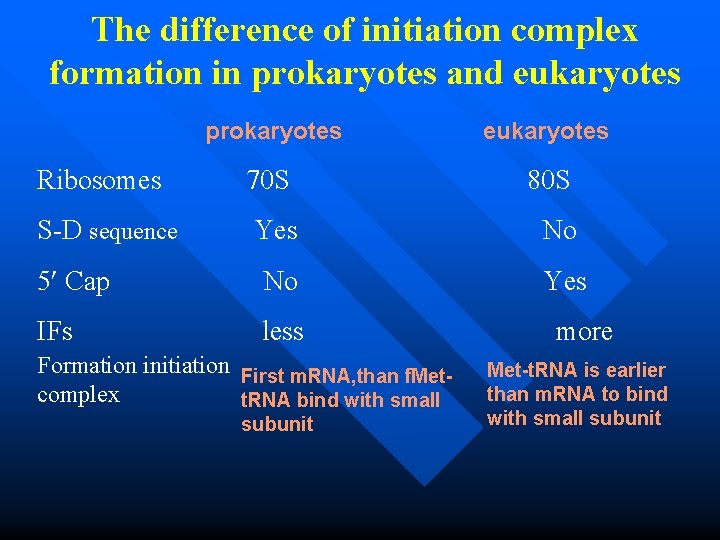
The difference of initiation complex formation in prokaryotes and eukaryotes prokaryotes eukaryotes Ribosomes 70 S 80 S S-D sequence Yes No 5 Cap No Yes IFs less more Formation initiation First m. RNA, than f. Metcomplex t. RNA bind with small subunit Met-t. RNA is earlier than m. RNA to bind with small subunit

Elongation in Prokaryotes After the placement of the initiator codon, polypeptide chain would elongate with the addition of amino acids. n For this reaction Elongation factors – EF-Tu, EF-Ts, EF-G (in prokaryotes), Energy source GTP are necessary for the elongation process. n

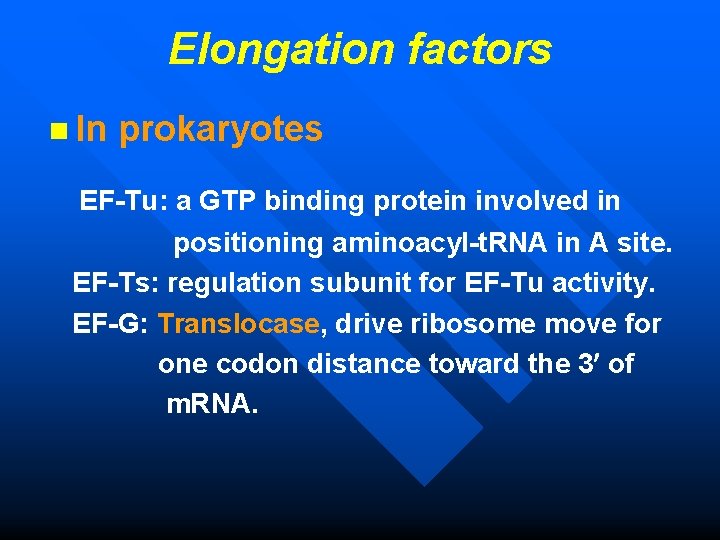
Elongation factors n In prokaryotes EF-Tu: a GTP binding protein involved in positioning aminoacyl-t. RNA in A site. EF-Ts: regulation subunit for EF-Tu activity. EF-G: Translocase, drive ribosome move for one codon distance toward the 3 of m. RNA.

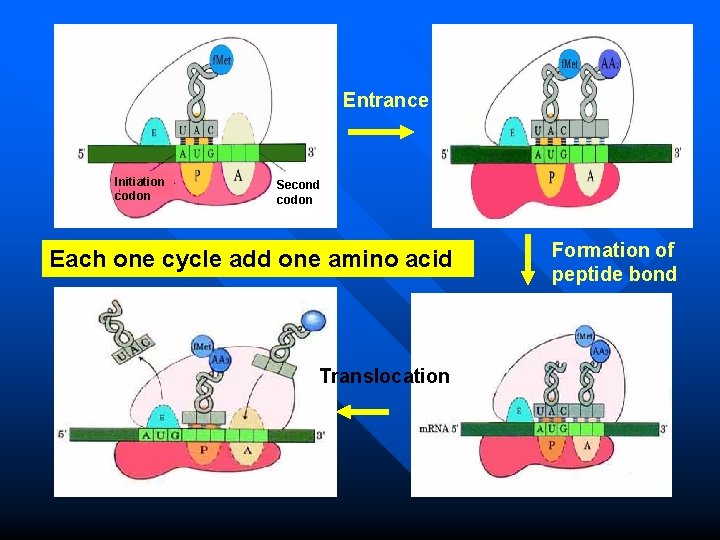
Elongation in Prokaryotes Three key steps: n Entrance (Registration) n Formation of peptide bond n Translocation These three steps form a cycle, each cycle leads to increase one amino acid to the polypeptide ---- Ribosomal cycle 149

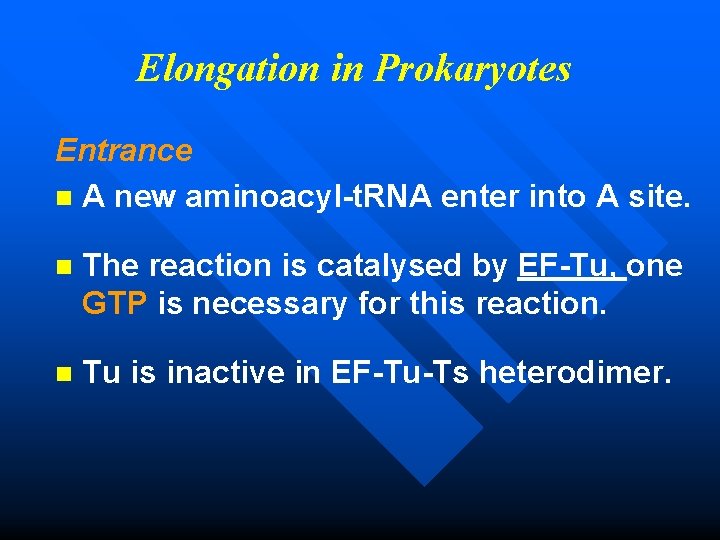
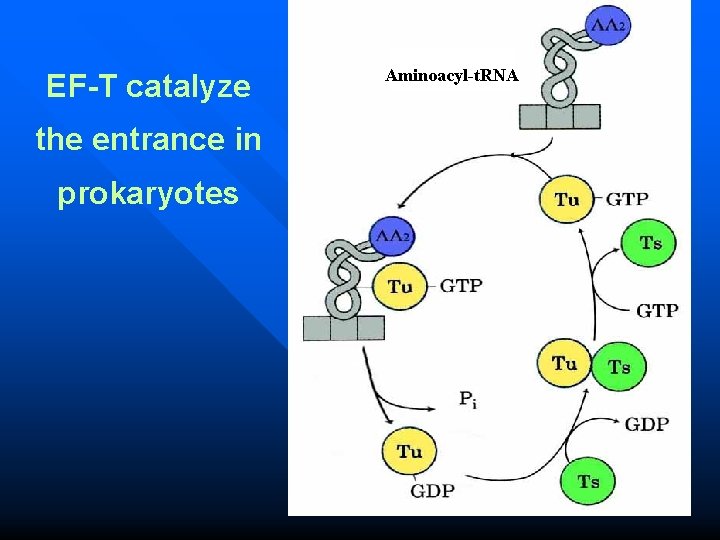
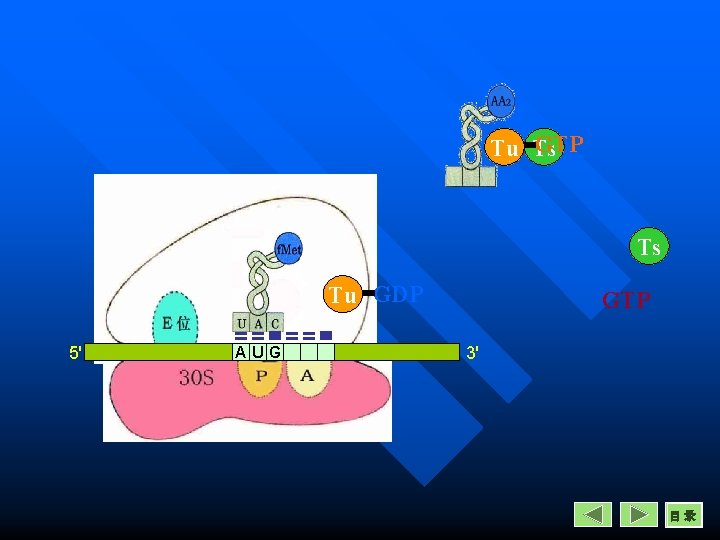
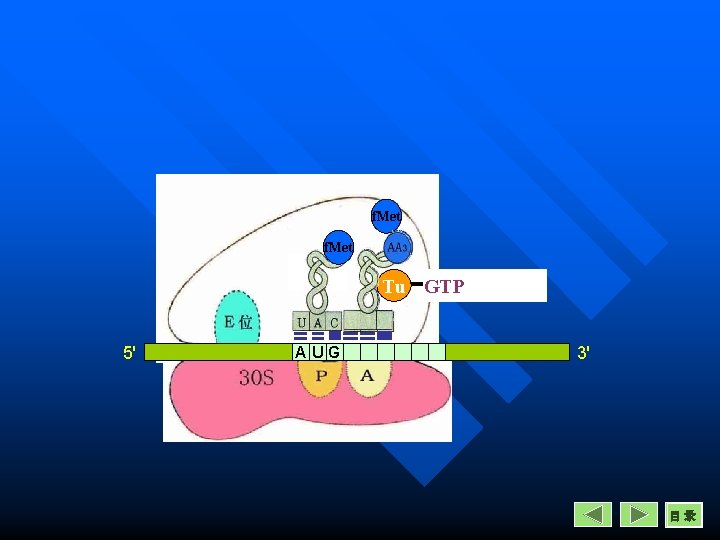
Elongation in Prokaryotes Entrance n A new aminoacyl-t. RNA enter into A site. n The reaction is catalysed by EF-Tu, one GTP is necessary for this reaction. n Tu is inactive in EF-Tu-Ts heterodimer.

EF-T catalyze the entrance in prokaryotes Aminoacyl-t. RNA

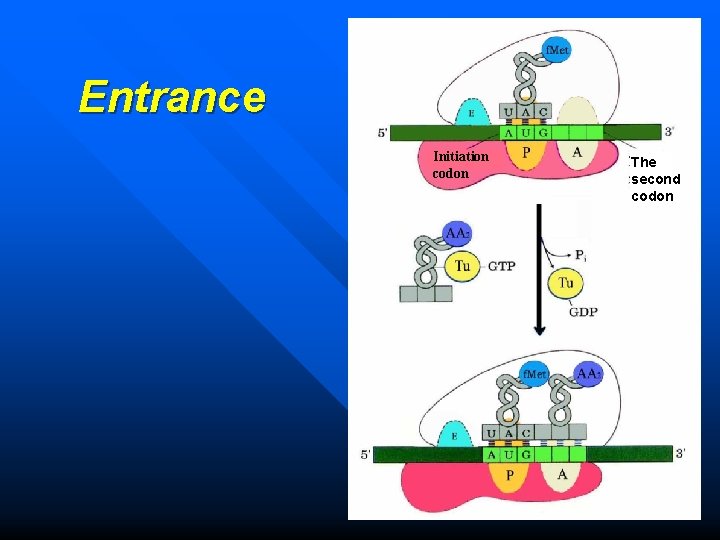
Entrance Initiation codon The second codon

GTP Tu Ts Ts Tu GDP 5' AUG GTP 3' 目录

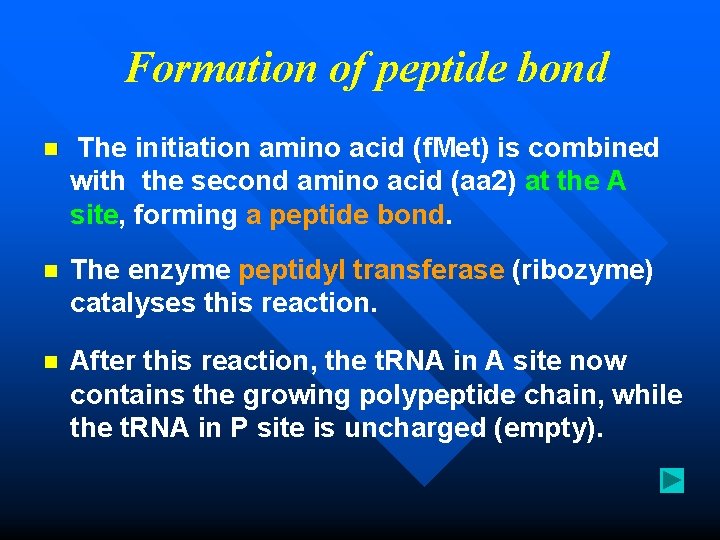
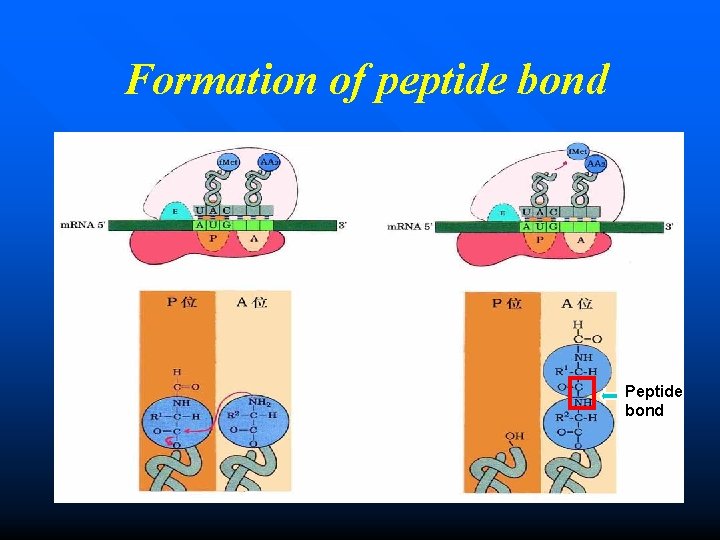
Formation of peptide bond n The initiation amino acid (f. Met) is combined with the second amino acid (aa 2) at the A site, forming a peptide bond. n The enzyme peptidyl transferase (ribozyme) catalyses this reaction. n After this reaction, the t. RNA in A site now contains the growing polypeptide chain, while the t. RNA in P site is uncharged (empty).

Formation of peptide bond Peptide bond

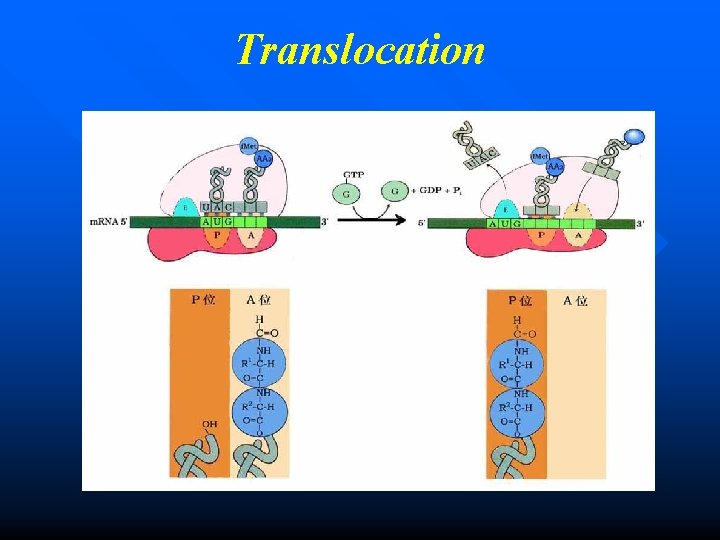
Translocation The ribosome now moves relative to m. RNA in the 5 3 direction one codon at one time. n The elongation factor G (EF-G, translocase) is necessary for this reaction. GTP is also needed. n Now the P site is occupied by the polytidyl-t. RNA comlex and the A site is vacant, which is ready to receive another new aminoacyl-t. RNA. n


f. Met Tu GTP 5' AUG 3' 目录

Entrance Initiation codon Second codon Each one cycle add one amino acid Translocation Formation of peptide bond

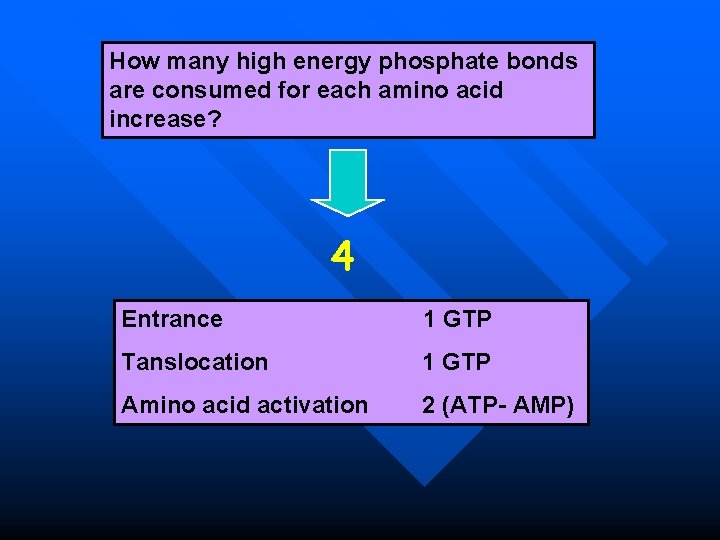
How many high energy phosphate bonds are consumed for each amino acid increase? 4 Entrance 1 GTP Tanslocation 1 GTP Amino acid activation 2 (ATP- AMP)

Elongation in eukaryotes P 209 Elongation process is similar with that in prokaryotes except different reaction system and e. EFs. n There is no E site in eukaryotes, uncharged t. RNA release directly from P site. n

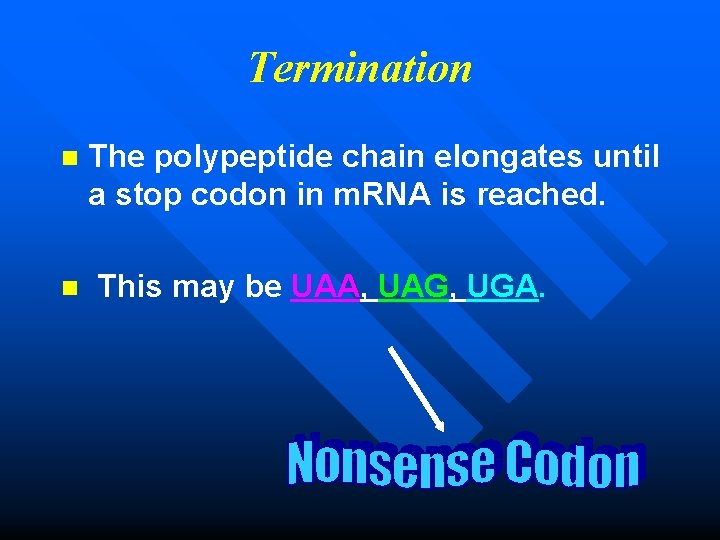
Termination n The polypeptide chain elongates until a stop codon in m. RNA is reached. n This may be UAA, UAG, UGA.

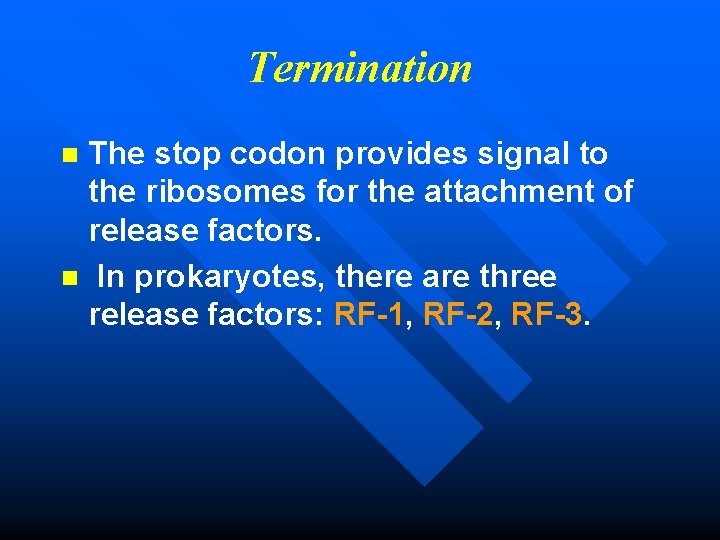
Termination The stop codon provides signal to the ribosomes for the attachment of release factors. n In prokaryotes, there are three release factors: RF-1, RF-2, RF-3. n

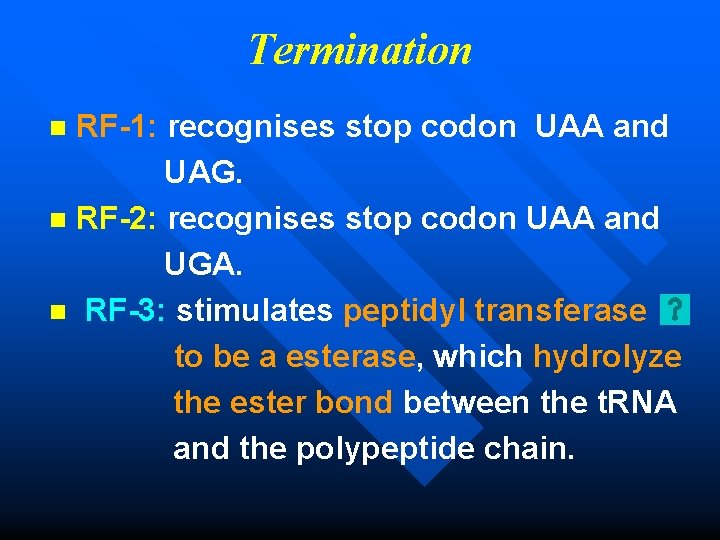
Termination RF-1: recognises stop codon UAA and UAG. n RF-2: recognises stop codon UAA and UGA. n RF-3: stimulates peptidyl transferase to be a esterase, which hydrolyze the ester bond between the t. RNA and the polypeptide chain. n

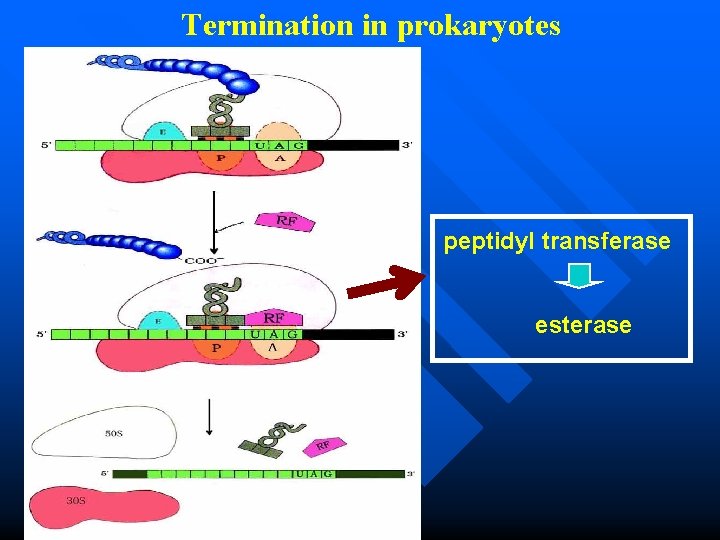
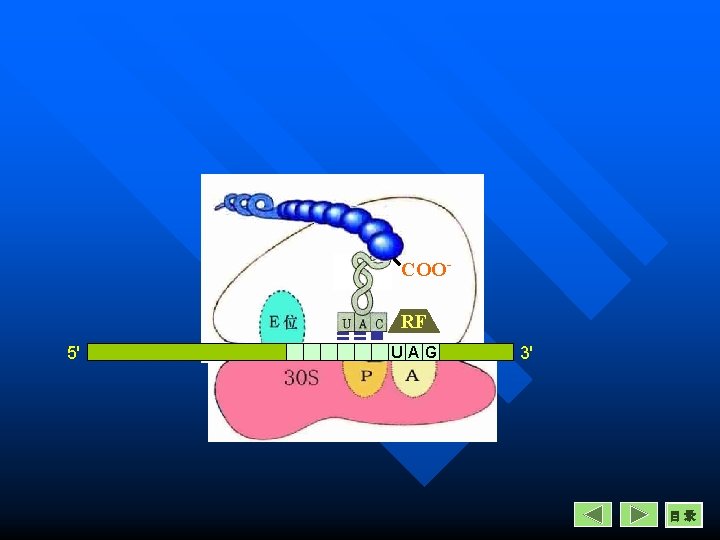
Termination Recognition of a stop codon n Polypeptide is released from peptidylt. RNA. (The release factors interact with the n peptidyl transferase and cause the hydrolysis of peptide chain at the P site) m. RNA is released from ribosome n Ribosome dissociation n

Termination in prokaryotes peptidyl transferase esterase

COORF 5' UAG 3' 目录

Termination in eukaryotes P 209 Termination process is similar with that in prokaryotes except different reaction system and e. RF. n Only one e. RF, which can recognize UAA, UAG, UGA and stimulate the peptidyl transferase to esterase to cleave the ester bond between polypeptide and t. RNA.


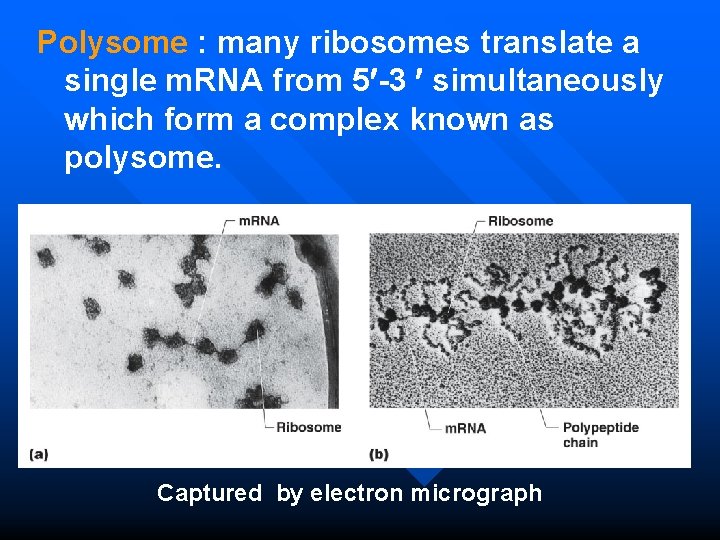
Polysome : many ribosomes translate a single m. RNA from 5 -3 simultaneously which form a complex known as polysome. Captured by electron micrograph

Polysome n n P 209 As one ribosome move along the m. RNA producing a polypeptide chain, a second ribosome can bind to vacant 5 end of m. RNA. Ribosomes are positioned on m. RNA at intervals of about 100 nucleotides. Polysome exists both in eukaryotes and prokaryotes. Ensuring the highly efficient use of the m. RNA.

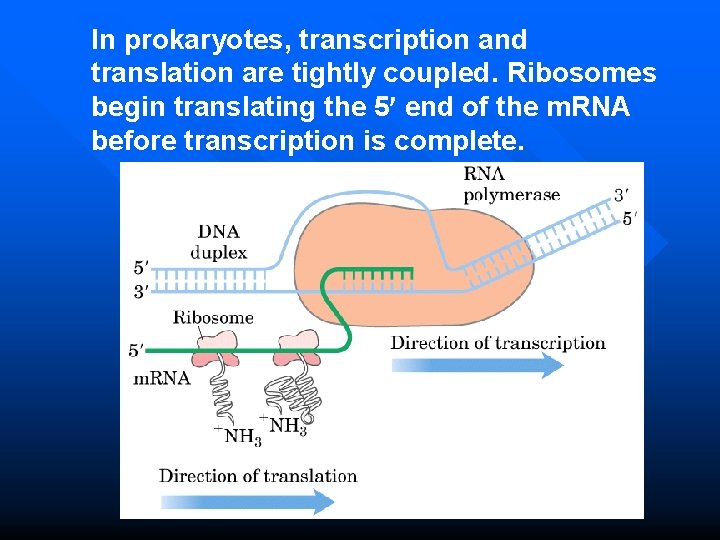
In prokaryotes, transcription and translation are tightly coupled. Ribosomes begin translating the 5 end of the m. RNA before transcription is complete.

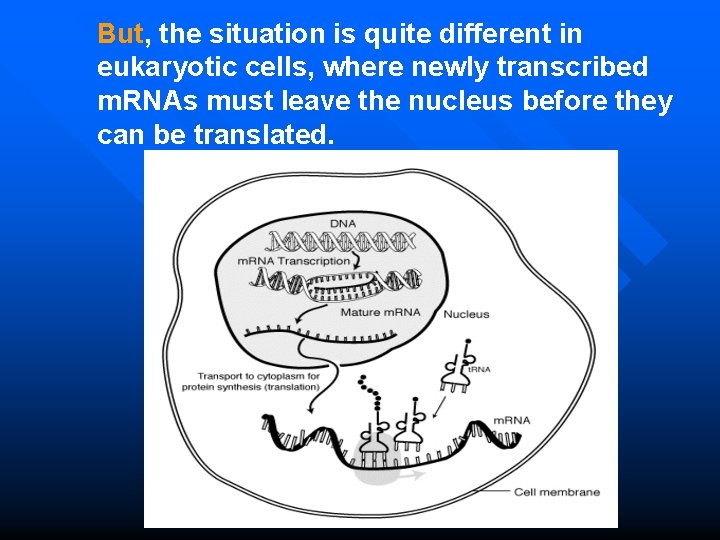
But, the situation is quite different in eukaryotic cells, where newly transcribed m. RNAs must leave the nucleus before they can be translated.

Protein modification It is estimated that the human proteome consists of ~300, 000 different proteins, or about 10 times more than the number of genes (? ? ) - protein modifications - differential splicing Protein modifications - different types, including co-translational and post-translational

Protein posttranslational modification No function The nascent polypeptide chain must be folded and processed into its biologically active form. n Some proteins may be posttranslationally modified. n These modifications are crucial to the functional capability of the final protein product. n

Protein Folding Process n During or after protein synthesis, the polypeptide progressively assumes its native conformation, with the formation of appropriate hydrogen bonds and van der Waals, ionic bonds, and hydrophobic interactions.

Protein Folding Process Protein folding need help from some molecules. n Molecular chaperones: Proteins that interact with partially folded or improperly folded peptides, facilitating correct folding pathways or providing microenvironments to direct their folding. n

Protein Folding Process The function of molecular chaperones n n Binding to short hydrophobic segments in unfolded polypeptides to prevent molten globule formation. Creating protected environment for unfolded or partly folded proteins, avioding them from degraded. Directing the refolding of protein partially unfolded as the consequence of stressful conditions. Promoting protein folding and unfolding.

Protein Folding Process Two major types of molecular chaperones Ribosome binding cheperone -- trigger factor -- nascent chain-associated complex (NAC) n Non-ribosome binding chaperone -- heat shock protein (HSP) -- chaperonins n

Protein posttranslational modification Primary structure modification -- N-terminal and C-terminal modification -- Amino acid residue modification -- Biological activity processing n Structural organization of proteins -- Subunit accumulation -- prosthetic group connection n

Primary structure modification N-terminal and C-terminal modification The first residue inserted in all polypeptides is Nformylmethionine (in prokaryotes) or methionine (in eukaryotes). n However, it is not the same in most natural proteins, the N-terminal methionine is not present in mature proteins. The formyl group, the N-terminal Met residue, and often additional N-terminal (and, in some cases, C-terminal) residues may be removed enzymatically in formation of the final functional protein. n

Primary structure modification N-terminal and C-terminal modification n . A number of other N-terminal modification occur, such as acetylation, Methylation and so on. ~80% eukaryotic cytosolic proteins are acetylated at their N-termini. Amidation of peptides (e. g. , hormones) sometimes occurs at the C-terminus.

Primary structure modification Amino acid residue modification n Glycosylation – glycosylation takes place in the ER (endoplasmic reticulum), golgi by a variety of enzymes – glycosylated proteins often found on the surface of cells or are secreted proteins. – glycosylation alters the properties of proteins, changing their stability, solubility and physical bulk. .

Primary structure modification Amino acid residue modification Phosphorylation – Serine, threonine and tyrosine can be phosphorylated by the catalysis of protein kinase. – phosphorylation can affect the activity and structure of proteins. – Too many examples to list: e. g. HSL activity is modulated by phosphorylation; cell-signalling molecules are best characterized.

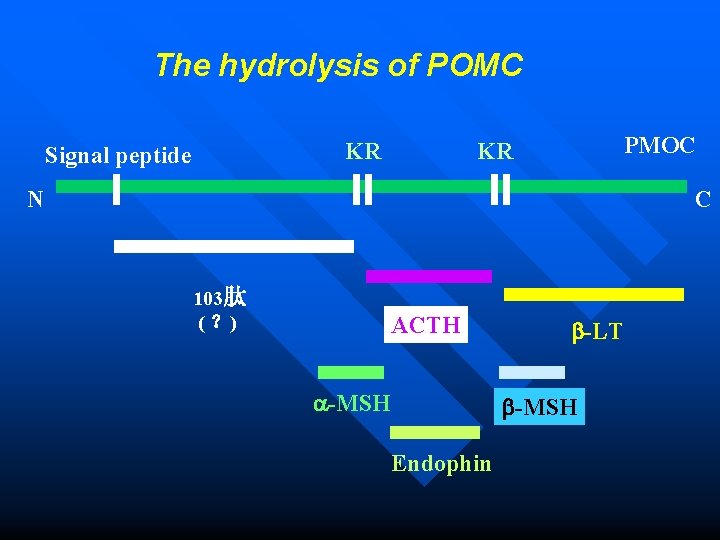
Primary structure modification Biological activity processing n n The peptide bond cleavages of prohormones or proenzymes can produce a more diverse of acivity proteins. POMC(pro-opiomelanocortin) with 256 amino acid residues can be hydrolyzed to form ACTH(adrenocorticotropic hormone), lipotropin( -LT), -MSH (melanocyte stimulating hormone), and endorphin and so on.

The hydrolysis of POMC KR Signal peptide PMOC KR N C 103肽 ( ?) ACTH -MSH -LT -MSH Endophin

Structural Organization of Proteins Subunit Accumulation n n Quaternary structure Some proteins are made of two or more peptides with tertiary structure by noncovalent bonds which allow to form oligomer, while the subunit interaction information is included in the sequence of amino acid in the polypeptides. e. g. Human hemoglobin is a 2 2 tetramer.

Structural Organization of Proteins Prosthetic group connection n Simple protein and conjugated protein. n Prosthetic groups are needed by conjugated proteins for function, such as lipoprotein.

Protein Targeting n The eukaryotic cell is made up of many structures, compartments, and organelles, each with specific functions that require distinct sets of proteins and enzymes. The proteins are synthesized on ribosomes in the cytosol, so how are they directed to their final cellular destinations? n

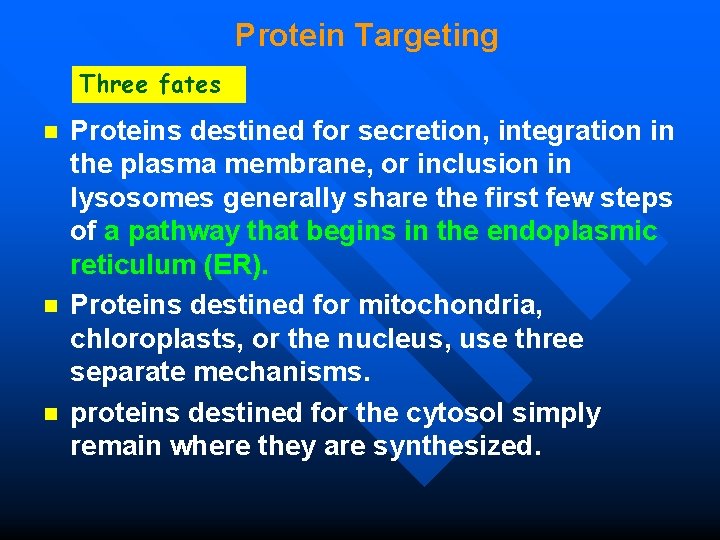
Protein Targeting Three fates n n n Proteins destined for secretion, integration in the plasma membrane, or inclusion in lysosomes generally share the first few steps of a pathway that begins in the endoplasmic reticulum (ER). Proteins destined for mitochondria, chloroplasts, or the nucleus, use three separate mechanisms. proteins destined for the cytosol simply remain where they are synthesized.

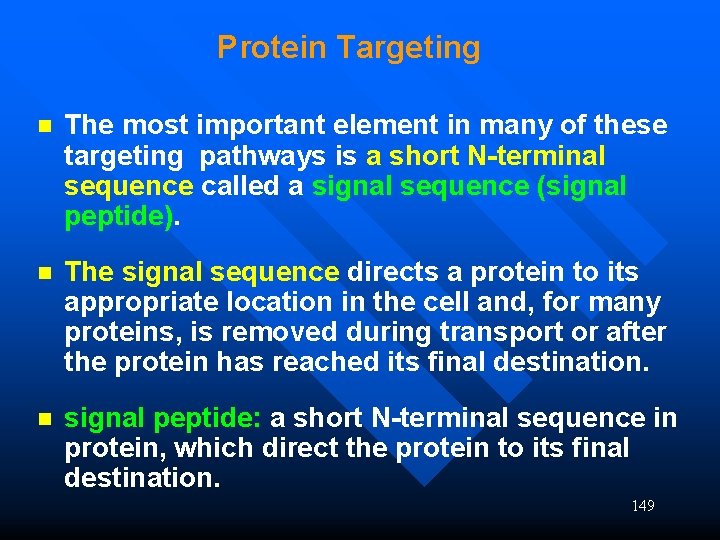
Protein Targeting n The most important element in many of these targeting pathways is a short N-terminal sequence called a signal sequence (signal peptide). n The signal sequence directs a protein to its appropriate location in the cell and, for many proteins, is removed during transport or after the protein has reached its final destination. n signal peptide: a short N-terminal sequence in protein, which direct the protein to its final destination. 149

Protein Targeting n n The fate of a targeted polypeptide depends on the location of the signal peptide or other signal sequences, which indicate information of targeting. Perhaps the best-characterized targeting system begins in the ER.

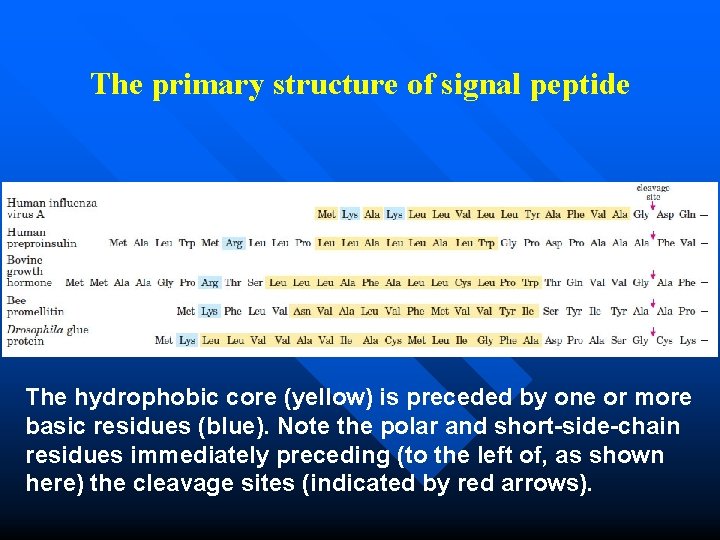
Signal peptide Signal sequences vary in length from 13 to 36 amino acid residues, but all have the following features: -- near the N terminus, a positively charged region composed of Arginine and lysine. -- a central hydrophobic region with leucine, isoleucine, etc. -- a short sequence at the C-terminal, that is relatively polar, typically having amino acid residues with short side chains (especially Ala), is accompanied by a cleavage site for signal peptide. n

The primary structure of signal peptide The hydrophobic core (yellow) is preceded by one or more basic residues (blue). Note the polar and short-side-chain residues immediately preceding (to the left of, as shown here) the cleavage sites (indicated by red arrows).

Protein Targeting n Many of secretary proteins and plasma membrane proteins reach their destination by transporting into ER for synthesizing and folding to functional proteins. n Then they are packed to secretary vesicles which are secreted into extracellular space through Golgi complex.

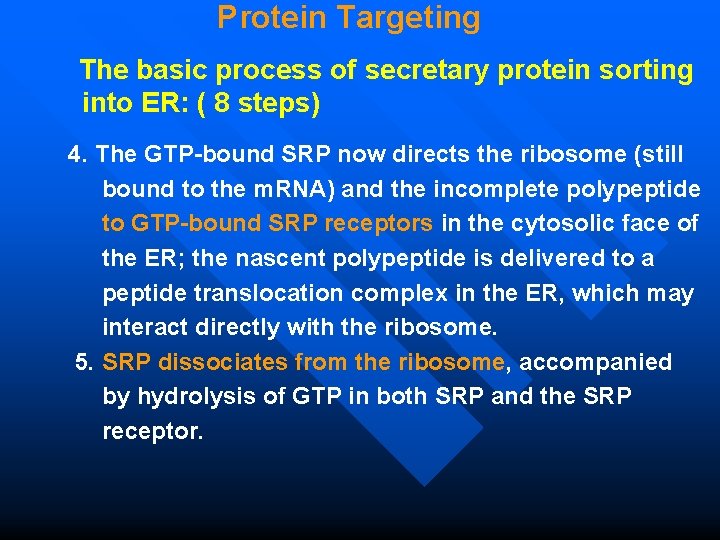
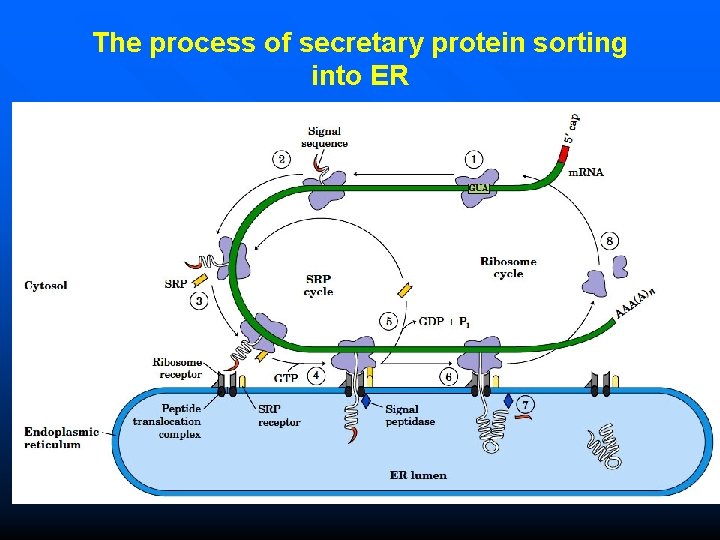
Protein Targeting The basic process of secretary protein sorting into ER: ( 8 steps) 1. The targeting pathway begins with initiation of protein synthesis on free ribosomes. 2. The signal sequence appears early in the synthetic process, because it is at the N-terminus, which as we have seen is synthesized first. 3. As it emerges from the ribosome, the signal peptide —and the ribosome itself— are bound by the large signal recognition particle (SRP); SRP then binds GTP and halts elongation of the polypeptide when it is about 70 amino acids long and the signal peptide has completely emerged from the ribosome.

Protein Targeting The basic process of secretary protein sorting into ER: ( 8 steps) 4. The GTP-bound SRP now directs the ribosome (still bound to the m. RNA) and the incomplete polypeptide to GTP-bound SRP receptors in the cytosolic face of the ER; the nascent polypeptide is delivered to a peptide translocation complex in the ER, which may interact directly with the ribosome. 5. SRP dissociates from the ribosome, accompanied by hydrolysis of GTP in both SRP and the SRP receptor.

Protein Targeting The basic process of secretary protein sorting into ER: ( 8 steps) 6. Elongation of the polypeptide now resumes, with the ATP-driven translocation complex feeding the growing polypeptide into the ER lumen until the complete protein has been synthesized. 7. The signal peptide is removed by a signal peptidase within the ER lumen. 8. The ribosome dissociates and is recycled.

The process of secretary protein sorting into ER

Antibiotics: inhibitor of protein synthesis n n n Protein synthesis is a central function in cellular physiology and is the primary target of many naturally occurring antibiotics and toxins. Except as noted, these antibiotics inhibit protein synthesis in bacteria. Caution must be exercised in their use, because some of the antibiotics affect human mitochondria.

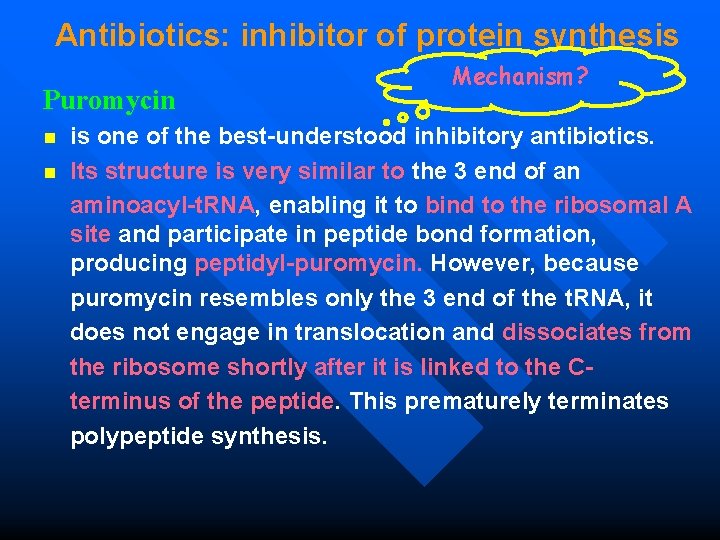
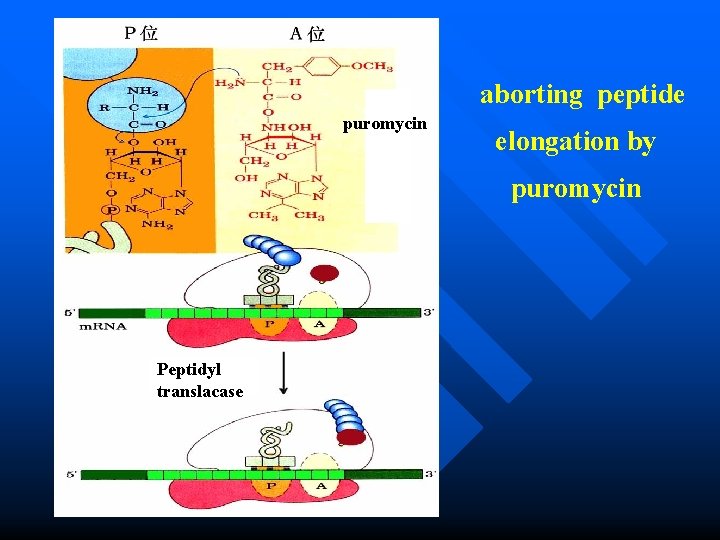
Antibiotics: inhibitor of protein synthesis Puromycin Mechanism? is one of the best-understood inhibitory antibiotics. n Its structure is very similar to the 3 end of an aminoacyl-t. RNA, enabling it to bind to the ribosomal A site and participate in peptide bond formation, producing peptidyl-puromycin. However, because puromycin resembles only the 3 end of the t. RNA, it does not engage in translocation and dissociates from the ribosome shortly after it is linked to the C terminus of the peptide. This prematurely terminates polypeptide synthesis. n

aborting peptide puromycin elongation by puromycin Peptidyl translacase

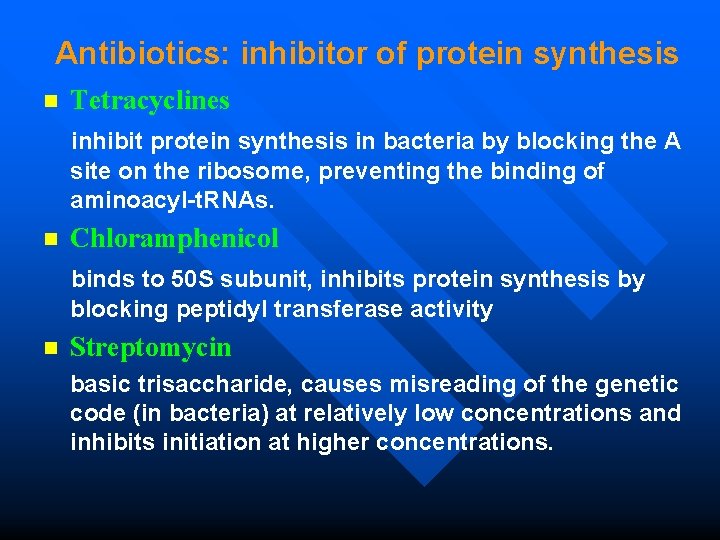
Antibiotics: inhibitor of protein synthesis Tetracyclines inhibit protein synthesis in bacteria by blocking the A n site on the ribosome, preventing the binding of aminoacyl-t. RNAs. Chloramphenicol binds to 50 S subunit, inhibits protein synthesis by n blocking peptidyl transferase activity n Streptomycin basic trisaccharide, causes misreading of the genetic code (in bacteria) at relatively low concentrations and inhibits initiation at higher concentrations.

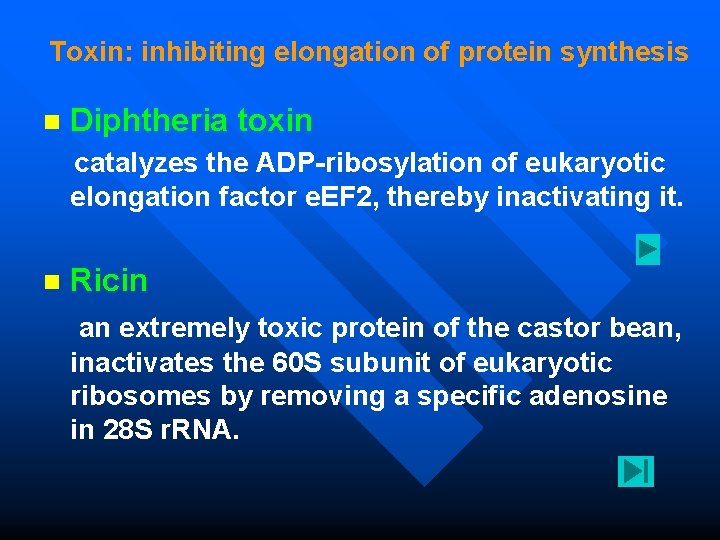
Toxin: inhibiting elongation of protein synthesis n Diphtheria toxin catalyzes the ADP-ribosylation of eukaryotic elongation factor e. EF 2, thereby inactivating it. Ricin an extremely toxic protein of the castor bean, n inactivates the 60 S subunit of eukaryotic ribosomes by removing a specific adenosine in 28 S r. RNA.

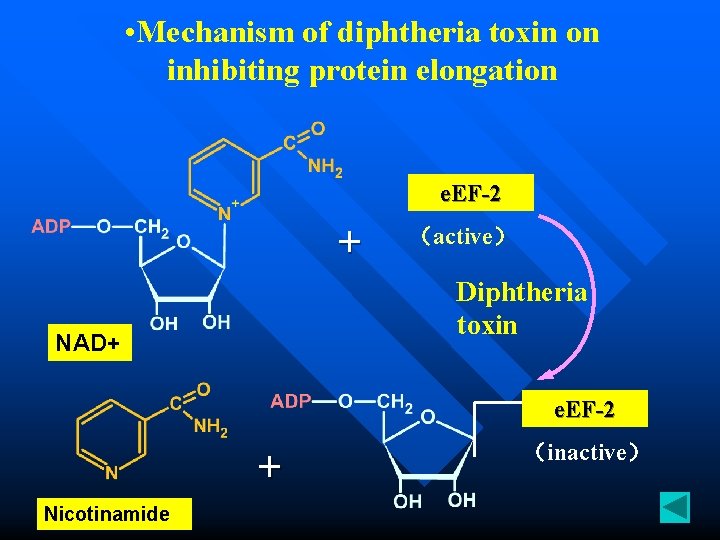
• Mechanism of diphtheria toxin on inhibiting protein elongation e. EF-2 + (active) Diphtheria toxin NAD+ e. EF-2 + Nicotinamide (inactive)

Spy activity Terrorist

Interferon Interferons are produced and liberated by the infection virus. n IFN- and IFN- have antiviral, antibacterial and anticancer activities. n Interferons play their antiviral role in two independent aspects. n

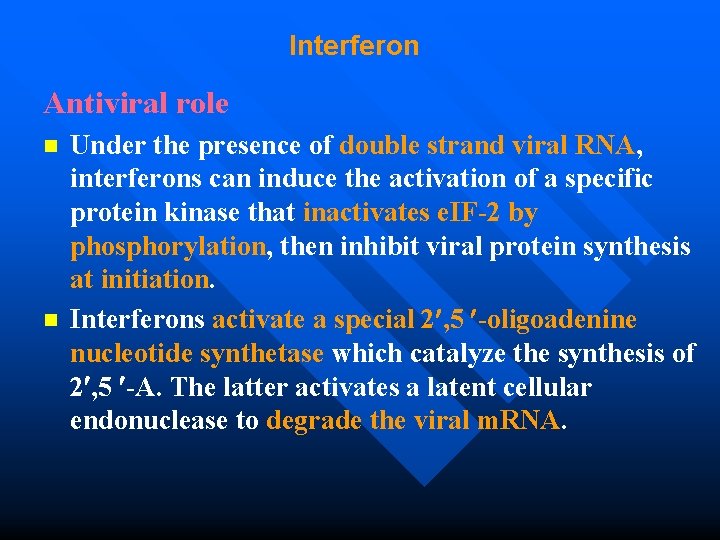
Interferon Antiviral role Under the presence of double strand viral RNA, interferons can induce the activation of a specific protein kinase that inactivates e. IF-2 by phosphorylation, then inhibit viral protein synthesis at initiation. n Interferons activate a special 2 , 5 -oligoadenine nucleotide synthetase which catalyze the synthesis of 2 , 5 -A. The latter activates a latent cellular endonuclease to degrade the viral m. RNA. n

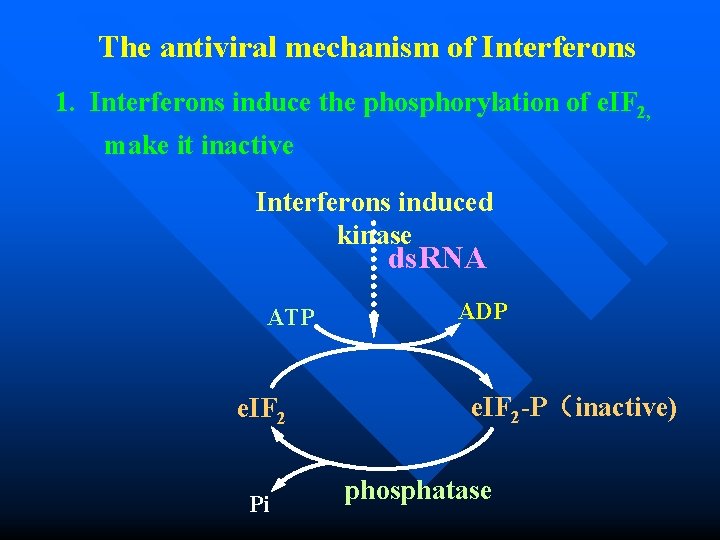
The antiviral mechanism of Interferons 1. Interferons induce the phosphorylation of e. IF 2, make it inactive Interferons induced kinase ds. RNA ATP e. IF 2 Pi ADP e. IF 2 -P(inactive) phosphatase

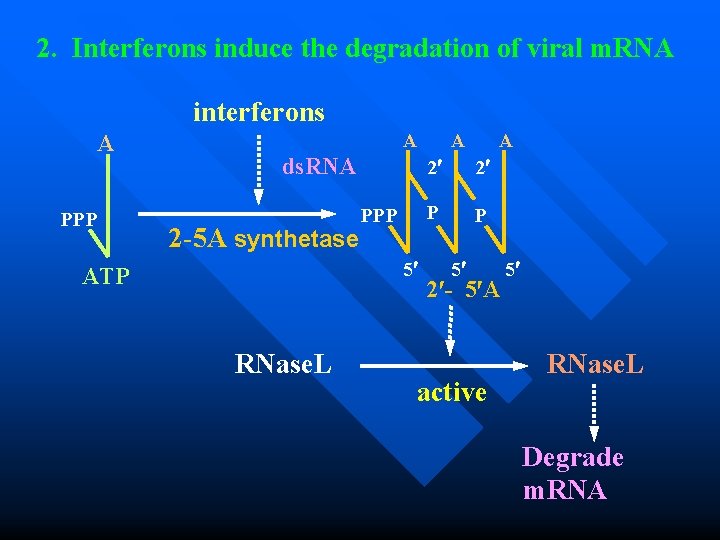
2. Interferons induce the degradation of viral m. RNA interferons A PPP A ds. RNA 2 -5 A synthetase PPP 5 ATP RNase. L A A 2 2 P P 5 2 - 5 A active 5 RNase. L Degrade m. RNA

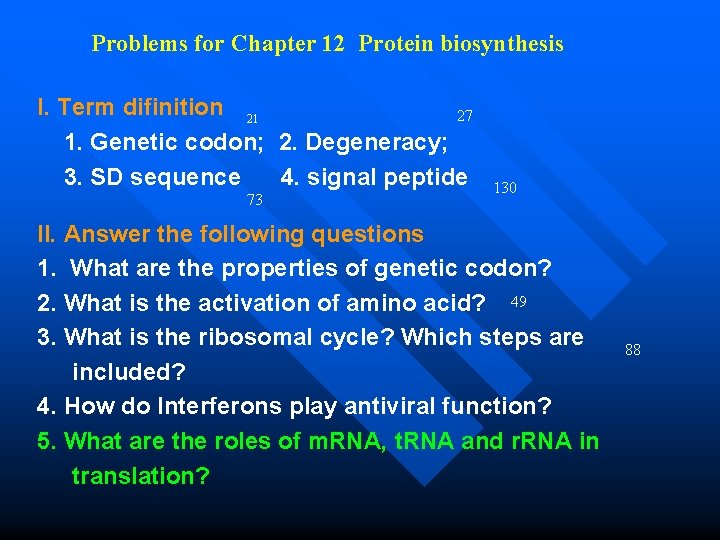
Problems for Chapter 12 Protein biosynthesis I. Term difinition 21 27 1. Genetic codon; 2. Degeneracy; 3. SD sequence 4. signal peptide 73 130 II. Answer the following questions 1. What are the properties of genetic codon? 2. What is the activation of amino acid? 49 3. What is the ribosomal cycle? Which steps are included? 4. How do Interferons play antiviral function? 5. What are the roles of m. RNA, t. RNA and r. RNA in translation? 88

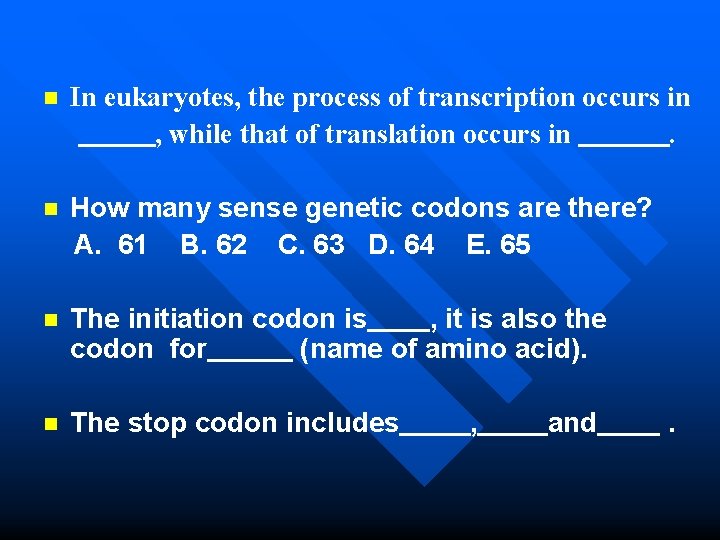
n In eukaryotes, the process of transcription occurs in , while that of translation occurs in. How many sense genetic codons are there? A. 61 B. 62 C. 63 D. 64 E. 65 n n The initiation codon is , it is also the codon for (name of amino acid). The stop codon includes , and . n

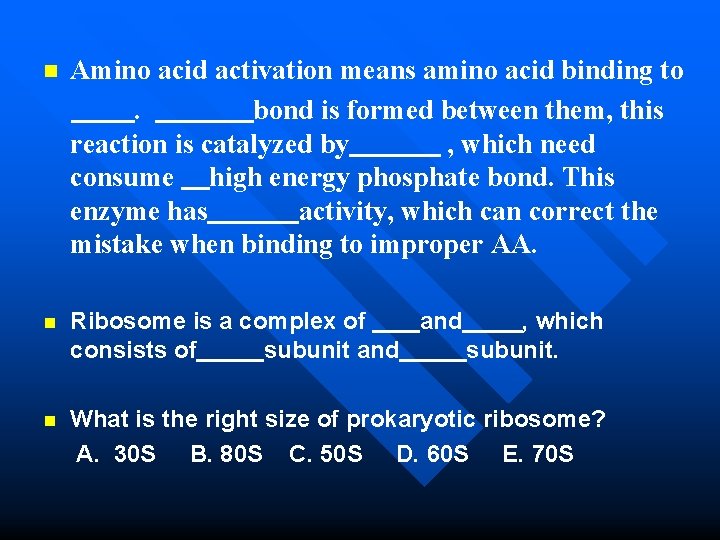
n Amino acid activation means amino acid binding to. bond is formed between them, this reaction is catalyzed by , which need consume high energy phosphate bond. This enzyme has activity, which can correct the mistake when binding to improper AA. n Ribosome is a complex of and , which consists of subunit and subunit. What is the right size of prokaryotic ribosome? A. 30 S B. 80 S C. 50 S D. 60 S E. 70 S n

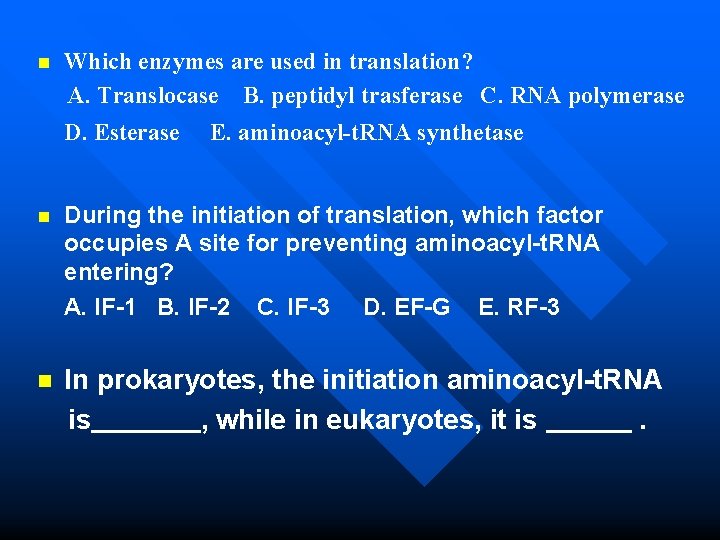
n Which enzymes are used in translation? A. Translocase B. peptidyl trasferase C. RNA polymerase D. Esterase E. aminoacyl-t. RNA synthetase During the initiation of translation, which factor occupies A site for preventing aminoacyl-t. RNA entering? A. IF-1 B. IF-2 C. IF-3 D. EF-G E. RF-3 n In prokaryotes, the initiation aminoacyl-t. RNA is , while in eukaryotes, it is . n

n Which structurs only exist in eukaryotes? A. SD sequence B. E site in ribosome C. 5 -cap D. 3 - poly. A tail E. monocistronic The elongation of translation is also called , which includes , and three steps. n Add one amono acid to polypeptide, at least high energy phosphate bonds are consumed. n During the initiation process in prokaryotes, there two recognitions ensure m. RNA precisely binding to small ribosomal subunit, one is recognition, another is recognition. n

Which stop codon can be recognized by release factor-1 in prokaryotes? A. AUG B. UAA C. UGA D. UAG E. UGG n n Signal peptide is usually near terminal of protein. Basic amino acid residues is near terminal of signal peptide. What is the mechanism of puromycin as a antibiotics? A. It can result in codon misreading B. It is a aminoacyl-t. RNA analog C. It can bind to A site and form peptidyl-puromycin D. It can abort peptide elongation E. It can prevent formation of initiation complex n
 Translation biology
Translation biology Hey hey bye bye
Hey hey bye bye Very bad to very good scale
Very bad to very good scale Used to express very large or very small numbers
Used to express very large or very small numbers Fewfewfewf
Fewfewfewf It is a very shallow skillet with very short sloping sides
It is a very shallow skillet with very short sloping sides Quantifiers for food
Quantifiers for food Rna protein synthesis
Rna protein synthesis Section 12-3 rna and protein synthesis
Section 12-3 rna and protein synthesis Rna protein synthesis
Rna protein synthesis Cell restaurant analogy
Cell restaurant analogy Protein synthesis splicing
Protein synthesis splicing Bbc bitesize protein synthesis
Bbc bitesize protein synthesis Protein synthesis and mutations
Protein synthesis and mutations Cookie monster analogy
Cookie monster analogy Guac wheel biology
Guac wheel biology Transfer rna
Transfer rna Protein synthesis
Protein synthesis Protein synthesis
Protein synthesis Protein synthesis and mutations
Protein synthesis and mutations Rna and protein synthesis study guide
Rna and protein synthesis study guide Protein synthesis story
Protein synthesis story Protein synthesis and mutations
Protein synthesis and mutations Protein synthesis animation mcgraw hill
Protein synthesis animation mcgraw hill Pap protein synthesis worksheet
Pap protein synthesis worksheet Protein synthesis
Protein synthesis Protein synthesis
Protein synthesis Protein synthesis ppt
Protein synthesis ppt Transcription and translation picture
Transcription and translation picture Protein synthesis inhibitor
Protein synthesis inhibitor Which best summarizes the process of protein synthesis?
Which best summarizes the process of protein synthesis? Concept map of protein synthesis
Concept map of protein synthesis Protein synthesis
Protein synthesis Protein synthesis scramble
Protein synthesis scramble Protein synthesis steps
Protein synthesis steps Dna nucleotide
Dna nucleotide Teste de ames
Teste de ames Sintese de proteinas na celula
Sintese de proteinas na celula Protein synthesis
Protein synthesis Protein synthesis
Protein synthesis Double stranded dna
Double stranded dna Protein synthesis
Protein synthesis Similarities between dna and rna
Similarities between dna and rna Dna rna protein synthesis homework #2 dna replication
Dna rna protein synthesis homework #2 dna replication Rna transfer
Rna transfer School is very important for
School is very important for Radio telex stations
Radio telex stations Communication plays a very important role in
Communication plays a very important role in Your smile is important to us
Your smile is important to us My dream university ppt
My dream university ppt Very important place
Very important place Very important dog
Very important dog In cement hardening process instants are very important
In cement hardening process instants are very important Very important word
Very important word Dont ask why why why
Dont ask why why why Newspaper article format
Newspaper article format Inverted pyramid in news writing
Inverted pyramid in news writing Least important to most important
Least important to most important Channel vs carrier proteins
Channel vs carrier proteins Protein-protein docking
Protein-protein docking Communicative translation example
Communicative translation example Cisco voice translation-rule
Cisco voice translation-rule Parent transformations
Parent transformations Semantic translation và communicative translation
Semantic translation và communicative translation All household materials are useful
All household materials are useful Why is the crucifixion important to christianity gcse
Why is the crucifixion important to christianity gcse Netball footwork rule
Netball footwork rule Why is communication important in the workplace
Why is communication important in the workplace Why experience is important
Why experience is important Strategic plan example
Strategic plan example Why is water important to living things
Why is water important to living things Important of reading
Important of reading Why is physical diversity important
Why is physical diversity important Why careful selection is important
Why careful selection is important Why are rivers important
Why are rivers important What is taxonomy and why is it important
What is taxonomy and why is it important What is selfawareness
What is selfawareness What is passover
What is passover What is advent
What is advent What to do during ramadan
What to do during ramadan Why are wetlands important
Why are wetlands important Zero-base forecasting
Zero-base forecasting Why is uml important
Why is uml important Why is tolerance important
Why is tolerance important Why is time management important
Why is time management important Why is food quality important
Why is food quality important Why is culture important
Why is culture important A polite expression of praise or admiration
A polite expression of praise or admiration Physical health triangle
Physical health triangle Why is genetic diversity important
Why is genetic diversity important Why is the sun important
Why is the sun important Why is keyboarding important
Why is keyboarding important The science of naming and classifying organisms
The science of naming and classifying organisms Why do writers use symbolism
Why do writers use symbolism Summarizing is a powerful
Summarizing is a powerful Organic social media
Organic social media Why is culture identity important
Why is culture identity important Filtration examples in everyday life
Filtration examples in everyday life Why is the greenhouse effect important
Why is the greenhouse effect important How to calculate net torque on a wheel
How to calculate net torque on a wheel Why break-even point is important
Why break-even point is important Fermentation process
Fermentation process Refusal skills meaning
Refusal skills meaning Why is phonological awareness important
Why is phonological awareness important Why is sawm important
Why is sawm important Why punctuation is important
Why punctuation is important Why is feedback important
Why is feedback important Why is cellular respiration important
Why is cellular respiration important Why is confidentiality important
Why is confidentiality important Onomatopoeia sound device examples
Onomatopoeia sound device examples Performance management activities
Performance management activities Parallel structure definition
Parallel structure definition Types of eclipses
Types of eclipses Aspect of community
Aspect of community Fear
Fear Why is cell division important
Why is cell division important Why is biodiversity important
Why is biodiversity important Marigolds by eugenia collier
Marigolds by eugenia collier What is ethics and why is it important
What is ethics and why is it important Why are situation and site factors important
Why are situation and site factors important Why is health education important
Why is health education important Interesting facts about river nile
Interesting facts about river nile How to calculate intake and output
How to calculate intake and output Why is patrick henry important
Why is patrick henry important What are the contributions of socrates
What are the contributions of socrates Benefits of hfle
Benefits of hfle Hasawa 1974
Hasawa 1974 Greenhouse effect in simple terms
Greenhouse effect in simple terms Fibers forensics
Fibers forensics Importance of wearing clothes
Importance of wearing clothes What is ecosystem biodiversity
What is ecosystem biodiversity It is used to drape the customer
It is used to drape the customer Why is dna important
Why is dna important Why is dignity important
Why is dignity important Why is performance management important?
Why is performance management important? On guard defending your faith with reason and precision
On guard defending your faith with reason and precision Why is culture identity important
Why is culture identity important Why is culture identity important
Why is culture identity important Why are gender roles important
Why are gender roles important Multimeter loopback adapter
Multimeter loopback adapter Forensic science chapter 1 observation skills
Forensic science chapter 1 observation skills Why are mobile systems increasingly important
Why are mobile systems increasingly important Chapter 23 milady review questions
Chapter 23 milady review questions Why is biodiversity important
Why is biodiversity important Why is genetic diversity important
Why is genetic diversity important Importance of marksmanship
Importance of marksmanship Why do tennis players need agility
Why do tennis players need agility 3 colligative properties
3 colligative properties Why is stewardship important
Why is stewardship important Why estuaries are important
Why estuaries are important Why are sacred texts important
Why are sacred texts important Why is a timetable important
Why is a timetable important What are study skills and why are they important
What are study skills and why are they important Why is the torah important
Why is the torah important Hospitality in the odyssey
Hospitality in the odyssey What are the 5 parameters in asl
What are the 5 parameters in asl What are study skills and why are they important
What are study skills and why are they important