Protein Synthesis Transcription and Translation Transcription Helicase enzyme

- Slides: 6

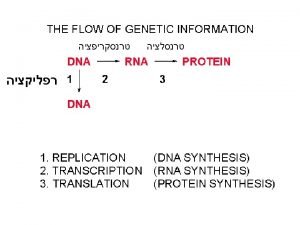
Protein Synthesis: Transcription and Translation

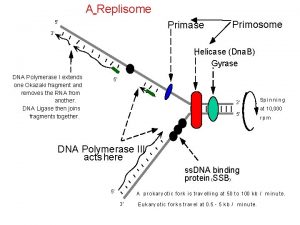
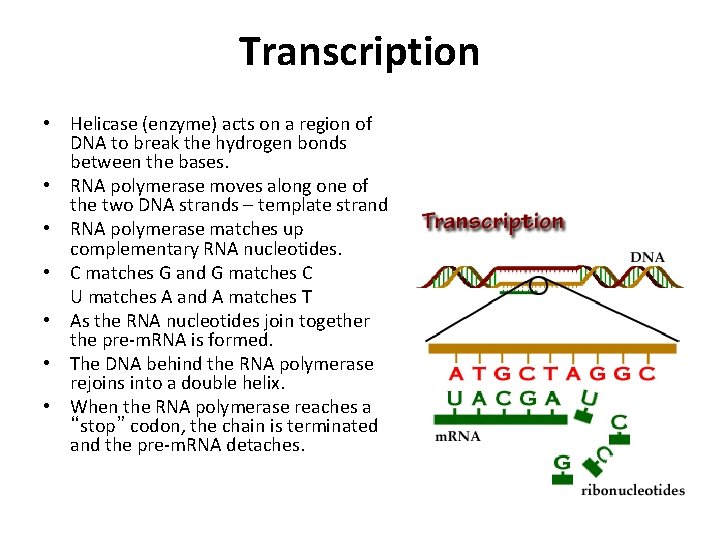
Transcription • Helicase (enzyme) acts on a region of DNA to break the hydrogen bonds between the bases. • RNA polymerase moves along one of the two DNA strands – template strand • RNA polymerase matches up complementary RNA nucleotides. • C matches G and G matches C U matches A and A matches T • As the RNA nucleotides join together the pre-m. RNA is formed. • The DNA behind the RNA polymerase rejoins into a double helix. • When the RNA polymerase reaches a “stop” codon, the chain is terminated and the pre-m. RNA detaches.

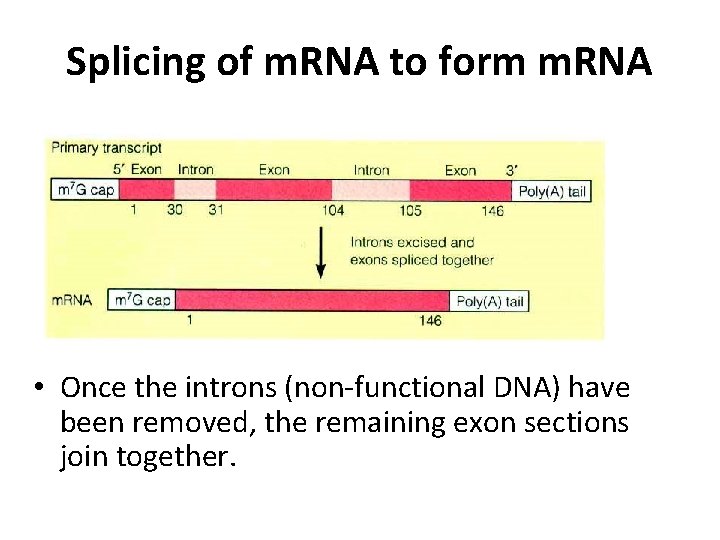
Splicing of m. RNA to form m. RNA • Once the introns (non-functional DNA) have been removed, the remaining exon sections join together.

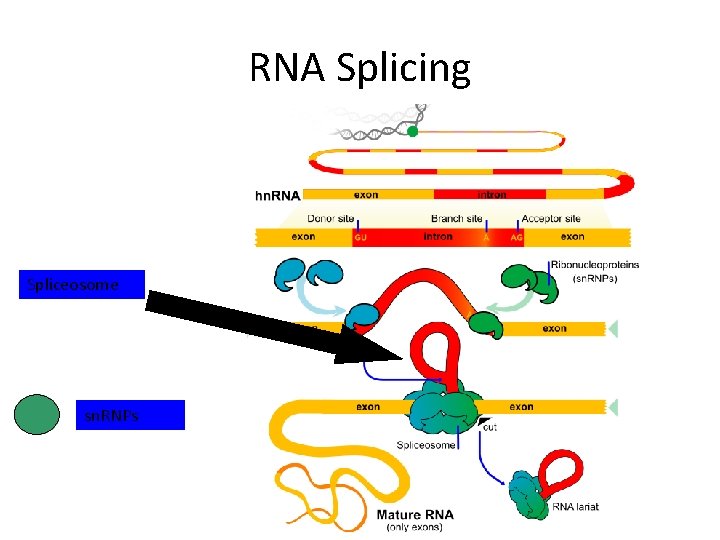
RNA Splicing Spliceosome sn. RNPs

Translation • m. RNA attaches to the ribosome at the “start” codon – AUG. • t. RNA with a complementary anticodon attaches to the m. RNA. • This t. RNA is attached to a specific amino acid – methionine • Two t. RNA molecules are bound to the m. RNA at the ribosome at any one time. • An enzyme and ATP are used to join the amino acids with a peptide bond. • The first t. RNA is released and can collect another amino acid. • The process is repeated until a “stop” codon is reached. • Many ribosomes can travel along the m. RNA at the same time – polysome.

How can you get different protein structures from a single gene • The m. RNA cane be formed from different combinations of exons • Which gives a different primary structure • This gives rise to a different secondary and tertiary structure since there will be different types and positions of intermolecular bonds (e. g. H bonds and disulfide bridges). This means that the proteins will have different 3 D shapes.