Protein Synthesis Inhibitors Protein Synthesis Inhibitors TETRACYCLINES MACROLIDES

Protein Synthesis Inhibitors

Protein Synthesis Inhibitors • • • TETRACYCLINES MACROLIDES CLINDAMYCIN CHLORAMPHENICOL STREPTOGRAMINS OXAZOLIDINONES
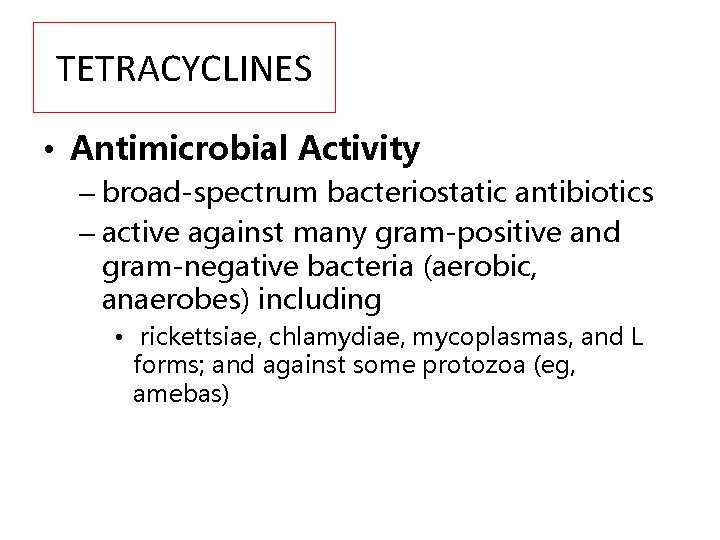
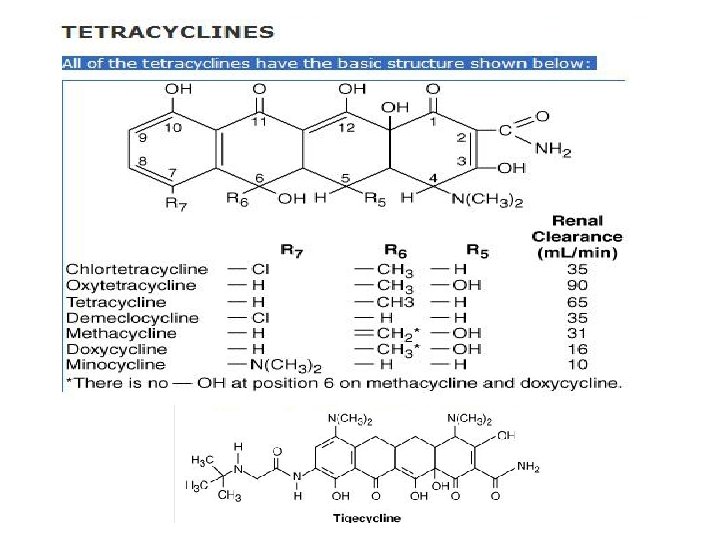
TETRACYCLINES • Antimicrobial Activity – broad-spectrum bacteriostatic antibiotics – active against many gram-positive and gram-negative bacteria (aerobic, anaerobes) including • rickettsiae, chlamydiae, mycoplasmas, and L forms; and against some protozoa (eg, amebas)
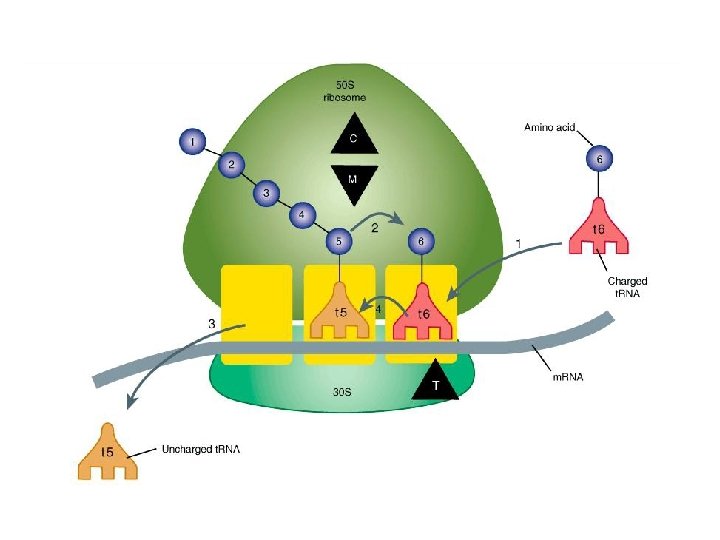
Mechanism of action • Tetracyclines enter microorganisms by – In part passive diffusion – in part by an energy-dependent process of active transport – bind reversibly to the 30 S subunit of the bacterial ribosome, – blocking the binding of aminoacyl-t. RNA to the acceptor site on the m. RNAribosome complex – prevents addition of amino acids to the growing peptide

TETRACYCLINES
Resistance • impaired influx or increased efflux by an active transport protein pump • ribosome protection due to production of proteins that interfere with tetracycline binding to the ribosome • enzymatic inactivation • The most important of these are production of an efflux pump and ribosomal protection

Pharmacokineti cs • Absorption is impaired divalent cations (Ca 2+, Mg 2+, Fe 2+) or Al 3+; by dairy products and antacids, which contain multivalent cations; and by alkaline p. H – except doxycycline and minocycline • Diffuse readily into body fluids – Minocycline reaches very high concentrations in tears and saliva
Pharmacokinet ics • Carbamazepine, phenytoin, barbiturates, and chronic alcohol – may shorten the half-life of doxycycline 50% by induction of hepatic enzymes • Tetracyclines are classified as: – short-acting (chlortetracycline, oxytetracycline), – intermediate-acting (demeclocycline and methacycline), – long-acting (doxycycline and minocycline)

Clinical Uses • • Mycoplasma pneumoniae, Chlamydiae Rickettsiae Spirochetes Helicobacter pylori Cholera tularemia, and brucellosis – (combination with aminoglycosides)

• Minocycline – eradicate the meningococcal carrier state • Demeclocycline – inhibits the action of antidiuretic hormone in the renal • Tigecycline – Staphylococcus aureus, MRSA, VRSA – enterococci, including vancomycinresistant strains – Proteus and P aeruginosa, however, are

Adverse Reactions • GASTROINTESTINAL ADVERSE EFFECTS – Nausea, vomiting, and diarrhea • Nausea, anorexia, and diarrhea can usually be controlled by administering with food • BONY STRUCTURES AND TEETH – bound to calcium deposited in newly formed bone or teeth in young children (under 8 years of age) – fluorescence, discoloration, and enamel dysplasia (fetal teeth)
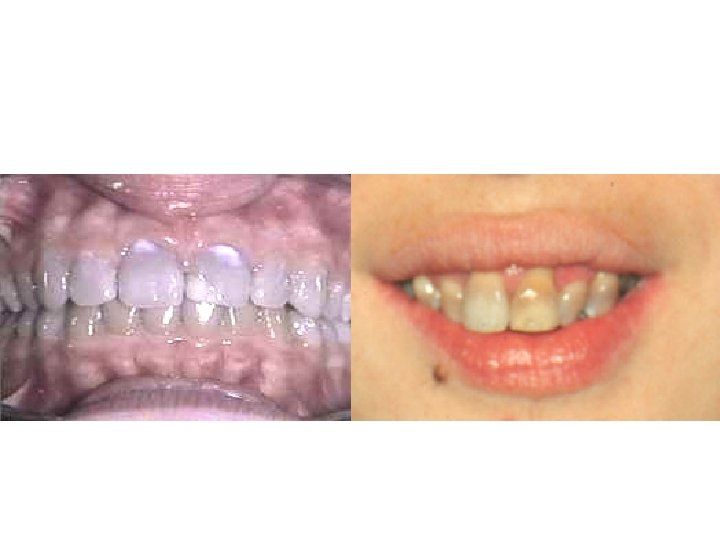
Adverse Reactions • KIDNEY TOXICITY – Renal tubular acidosis (fanconi syndrome) • LOCAL TISSUE TOXICITY – venous thrombosis • PHOTOSENSITIZATION – sensitivity to sunlight or ultraviolet light • Demeclocycline • VESTIBULAR REACTIONS – Dizziness, vertigo, nausea, and vomiting

MACROLIDES Macrocyclic lactone ring
Mechanism of action • binding to the 50 S ribosomal RNA, which blocks the aminoacyl translocation reaction and formation of initiation complexes • Bacteriostatic • Bacteriocide (higher doses) • Activity is enhanced at alkaline p. H

Resistance • reduced permeability of the cell membrane or active efflux • production of esterases (by Enterobacteriaceae) that hydrolyze macrolides • modification of the ribosomal binding site (so-called ribosomal protection) by chromosomal mutation or by a macrolide-inducible or constitutive methylase

MACROLIDE S • Erythromycin – Antimicrobial Activity • pneumococci, streptococci, staphylococci, and corynebacteria, Mycoplasma, legionella, Chlamydia trachomatis, C pneumoniae, helicobacter, listeria • The antibacterial action of erythromycin may be inhibitory or bactericidal, particularly at higher concentrations, for susceptible organisms

Clinical Uses • Drug of choice in corynebacterial infections, chlamydial infections and in community acquired pneumonia • Useful as a penicillin substitute in penicillin allergic individuals with infections caused by staphylococci, streptococci, or pneumococci.

Adverse Reactions • GASTROINTESTINAL EFFECTS – Anorexia, nausea, vomiting, and diarrhea • LIVER TOXICITY – acute cholestatic hepatitis (fever, jaundice, impaired liver function) – allergic reactions include fever, eosinophilia, and rashes

DRUG INTERACTIONS • inhibit cytochrome P 450 enzymes – theophylline, oral anticoagulants, cyclosporine, methylprednisolone, oral digoxin

CLARITHROMYCIN • Clarithromycin has improved acid stability and oral absorption compared with erythromycin • Mycobacterium avium • M leprae • Toxoplasma gondii

AZITHROMYC IN • penetrates into most tissues (except cerebrospinal fluid) and phagocytic cells • The drug is slowly released from tissues (tissue half-life of 2– 4 days) • elimination half-life is 3 days • administered 1 hour before or 2 hours after meals • azithromycin does not inactivate

AZITHROMYCI N • M avium • T gondii, • staphylococci, streptococci (less than erythromycin) • H influenzae • Chlamydia

KETOLIDES • Telithromycin – poor substrates for efflux pump-mediated resistance – bind to ribosomes of some bacterial species with higher affinity than macrolides – community-acquired bacterial pneumonia – acute exacerbations of chronic bronchitis – sinusitis – streptococcal pharyngitis
CLINDAMYC IN • Mechanism of action – The binding site for clindamycin on the 50 S subunit – inhibits protein synthesis • interfer with the formation of initiation complexes and with aminoacyl translocation reactions. • Antibacterial Activity – Streptococci, staphylococci, and pneumococci – Bacteroides species and other
Resistanc e • mutation of the ribosomal receptor site • modification of the receptor by a constitutively expressed methylase • enzymatic inactivation of clindamycin • Gram-negative aerobic species are intrinsically resistant because of poor permeability of the outer membrane
Pharmacokineti cs • penetrates well into most tissues • brain and cerebrospinal fluid being important exceptions • It penetrates well into abscesses and is actively taken up and concentrated by phagocytic cells

Clinical Uses • skin and soft-tissue infections • anaerobic infection caused by bacteroides • infections originating in the female genital tract, eg, septic abortion and pelvic abscesses • Prophylaxis of endocarditis (valvular heart disease) • AIDS related – Toxoplasma of the brain

Adverse Effects • diarrhea, nausea • Superinfection (colitis due to Clostridium difficile)
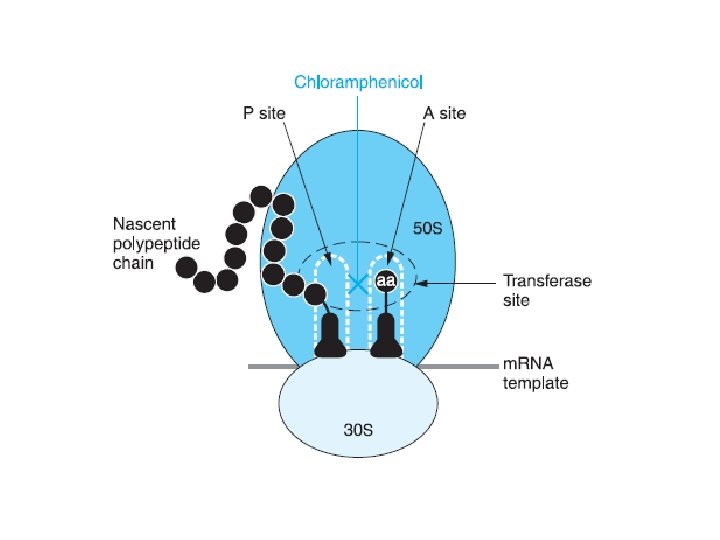
CHLORAMPHENI COL • Antimicrobial Activity – binds reversibly to the 50 S subunit of the bacterial ribosome – inhibit the peptidyl transferase step of protein synthesis – Chloramphenicol is a bacteriostatic broad -spectrum antibiotic • aerobic and anaerobic gram-positive and gram-negative organisms.

Resistanc e • selection of mutants that are less permeable to the drug • production of chloramphenicol acetyltransferase, a plasmid-encoded enzyme that inactivates the drug
Pharmacokineti cs • completely absorbed (oral) • widely distributed to virtually all tissues and body fluids (CNS & CSF) • Inactivated by conjugation with glucuronic acid

Clinical Uses • serious rickettsial infections – typhus and Rocky Mountain spotted fever – alternative to a -lactam antibiotic for treatment of meningococcal meningitis and pneumococci

Adverse Reactions • GASTROINTESTINAL DISTURBANCES – nausea, vomiting, and diarrhea • BONE MARROW DISTURBANCES – dose-related reversible suppression of red cell production – Aplastic anemia (idiosyncratic reaction) • TOXICITY FOR NEWBORN INFANTS – gray baby syndrome • vomiting, flaccidity, hypothermia, gray color, shock, and collapse
INTERACTION WITH OTHER DRUGS • inhibits hepatic microsomal enzymes – phenytoin, tolbutamide, chlorpropamide, and warfarin • bactericidal drugs such as penicillins or aminoglycosides

STREPTOGRAMIN S • • • Quinupristin-dalfopristin quinupristin, a streptogramin B dalfopristin, a streptogramin A— 30: 70 ratio bactericidal for most organisms except Enterococcus faecium
ANTIBACTERIAL ACTIVITY • gram-positive cocci – multidrug-resistant strains of streptococci • penicillin-resistant strains of S pneumoniae, methicillin-susceptible and resistant strains of staphylococci, and E faecium (but not E faecalis).
Mechanism of action • bind to the 50 S ribosomal subunit • Quinupristin inhibits polypeptide elongation • Dalfopristin binds at an adjacent site, changing the conformation of the 50 S ribosome – synergistically enhances the binding of quinupristin

Resistance • Resistance is due to modification of the quinupristin binding site (MLS-B type), enzymatic inactivation of dalfopristin, or efflux

Adverse Reactions • pain at the infusion site, and an arthralgia-myalgia syndrome • Quinupristin and dalfopristin significantly inhibit CYP 3 A 4 – Drug interactions • warfarin, diazepam, astemizole, terfenadine, cisapride, nonnucleoside reverse transcriptase inhibitors, and cyclosporine

OXAZOLIDINON ES • Linezolid – Linezolid is 100% bioavailable after oral administration – prevention formation of the ribosome complex that initiates protein synthesis – bind to 50 S subunit – Resistance is caused by mutation of the linezolid binding site on 23 S ribosomal RNA

ANTIBACTERIAL ACTIVITY • vancomycin-resistant E faecium infections • nosocomial pneumonia • community-acquired pneumonia • skin infections, complicated or uncomplicated • Off-lable use – Nocardia – Multidrug resistant tuberculosis

Adverse Reaction • • Thrombocytopenia Anemia and neutropenia peripheral neuropathy lactic acidosis have been reported with prolonged courses of linezolid

MUPIROCI N • active against many gram-positive and selected gram-negative bacteria. • It has good activity against S. pyogenes and methicillin-susceptible and methicillin resistant strains of S. aureus
Mechanism of Action • Mupirocin inhibits bacterial protein synthesis by reversible inhibition of ILe t. RNA synthase • There is no cross-resistance with other antibiotic classes • Resistance – mutations of the gene encoding ILe t. RNA synthase or an extra chromosomal copy of a gene encoding a modified ILe t. RNA synthase

Dosage Form • Mupirocin is available as a 2% cream or ointment for dermatologic use and as a 2% ointment for intranasal use
- Slides: 53