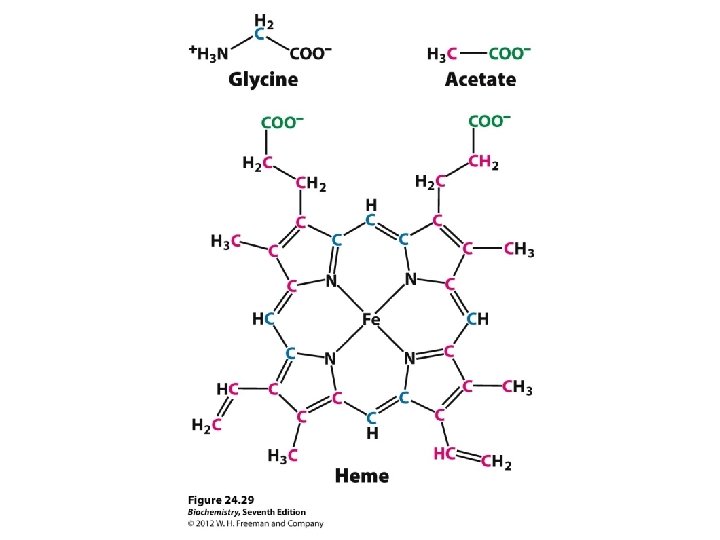
Protein structure Primary structure summary Amino acids joined


- Slides: 41

Protein structure

Primary structure, summary • Amino acids joined together by peptide bonds • Order of amino acids determines structure • Properties of peptide bonds: – Planar – Rotation around Cα – Steric hindrance

Secondary structure

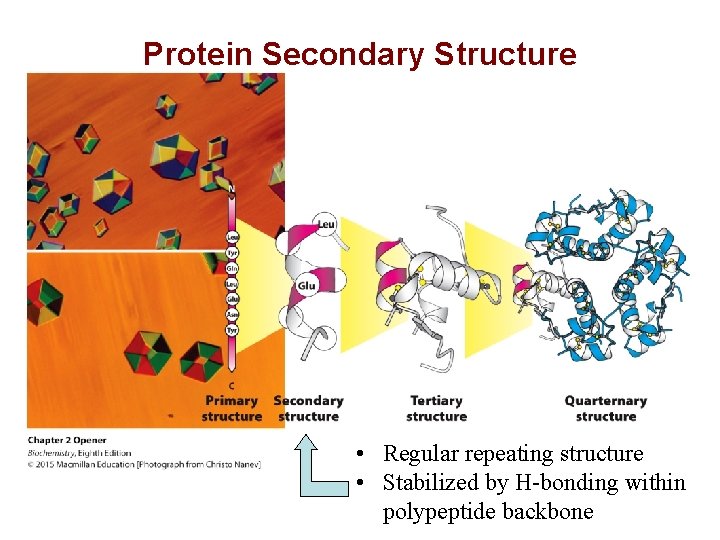
Protein Secondary Structure • Regular repeating structure • Stabilized by H-bonding within polypeptide backbone

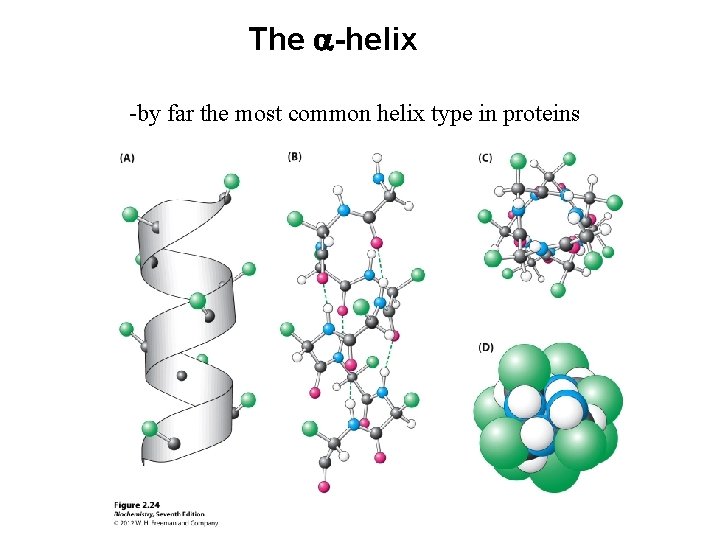
The a-helix -by far the most common helix type in proteins

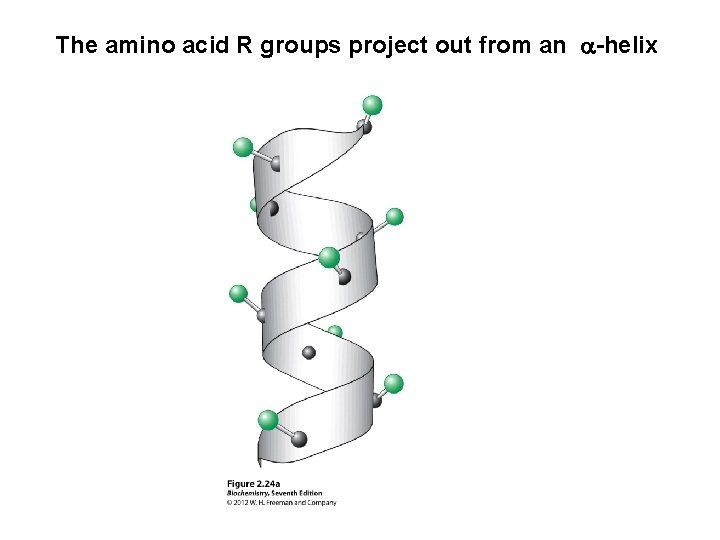
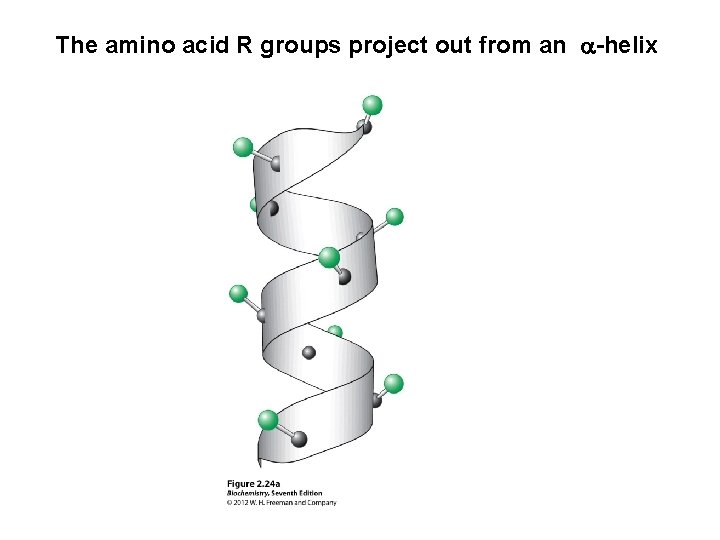
The amino acid R groups project out from an a-helix

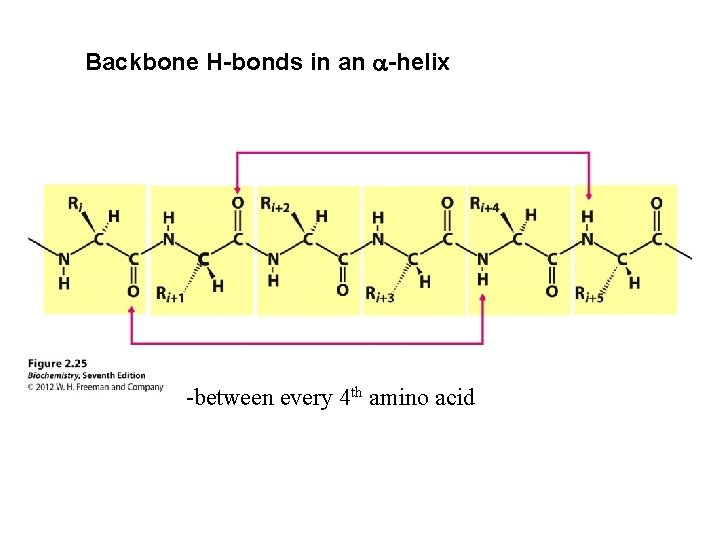
Backbone H-bonds in an a-helix -between every 4 th amino acid

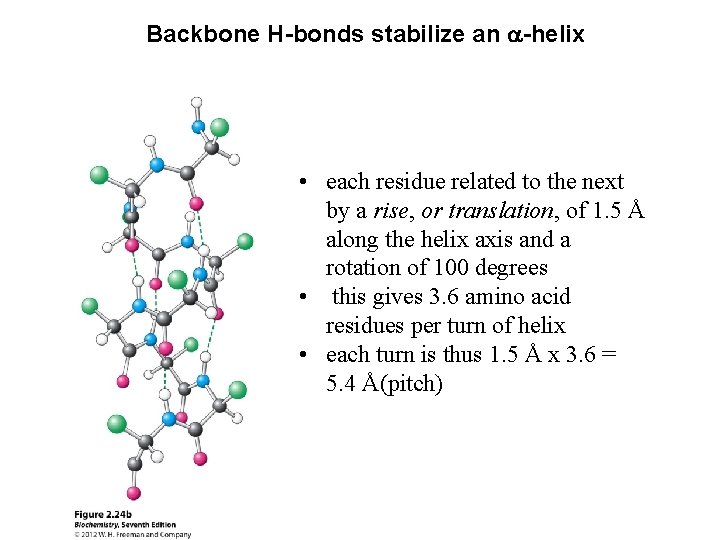
Backbone H-bonds stabilize an a-helix • each residue related to the next by a rise, or translation, of 1. 5 Å along the helix axis and a rotation of 100 degrees • this gives 3. 6 amino acid residues per turn of helix • each turn is thus 1. 5 Å x 3. 6 = 5. 4 Å(pitch)

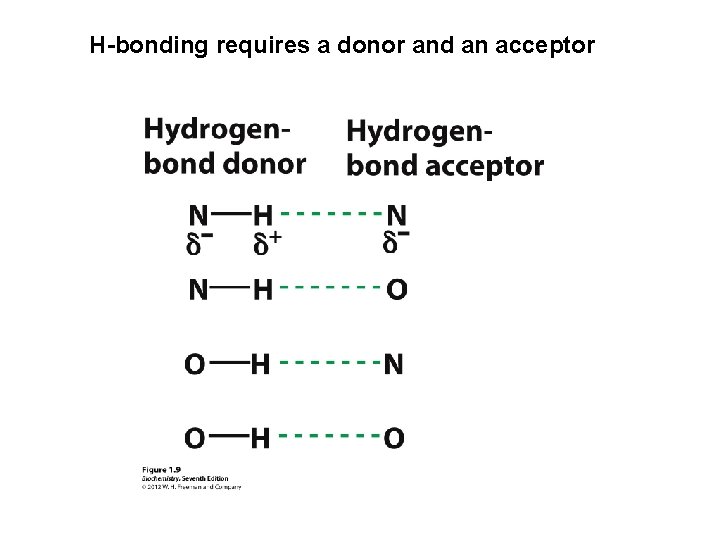
H-bonding requires a donor and an acceptor

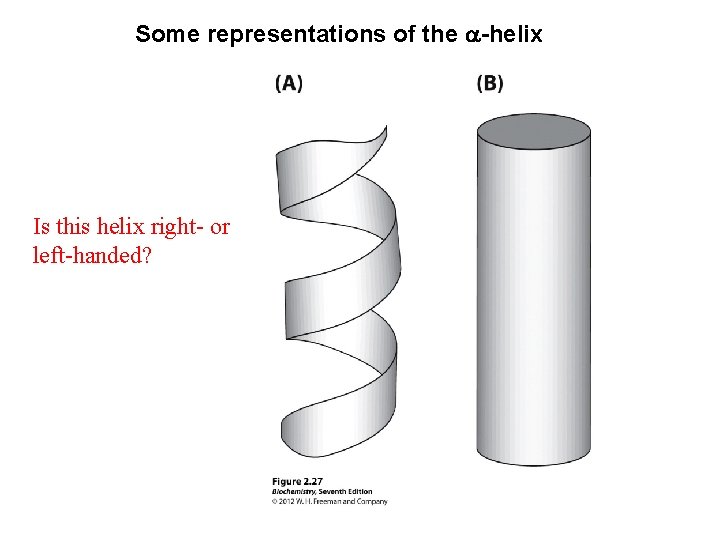
Some representations of the a-helix Is this helix right- or left-handed?

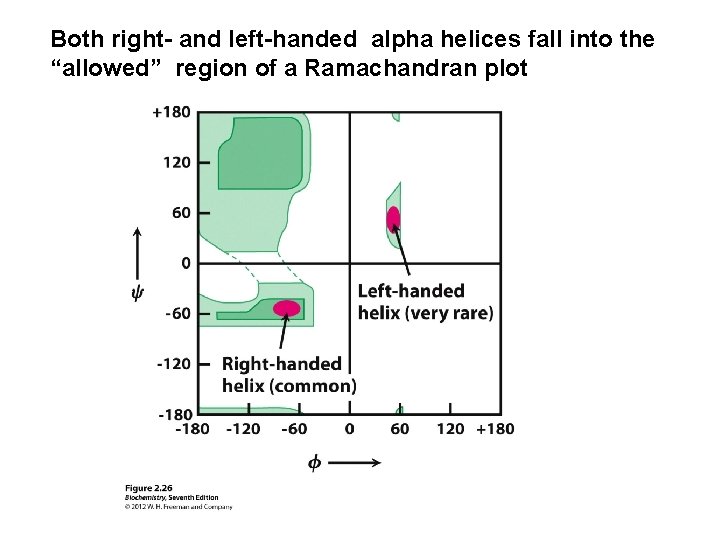
Both right- and left-handed alpha helices fall into the “allowed” region of a Ramachandran plot

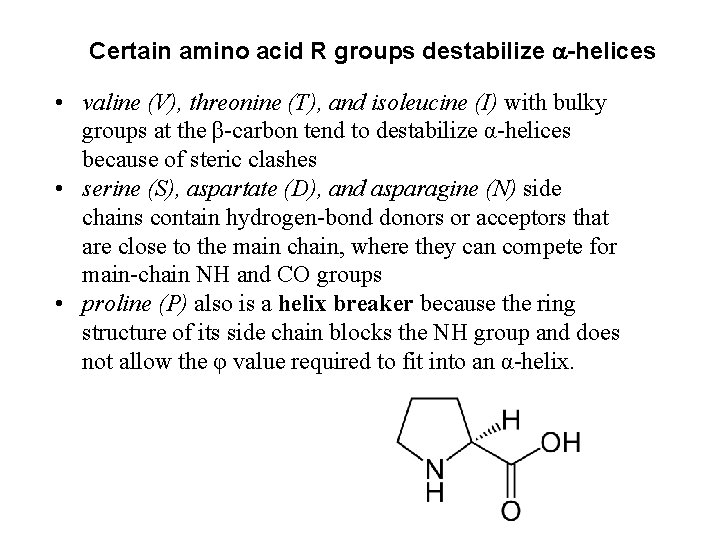
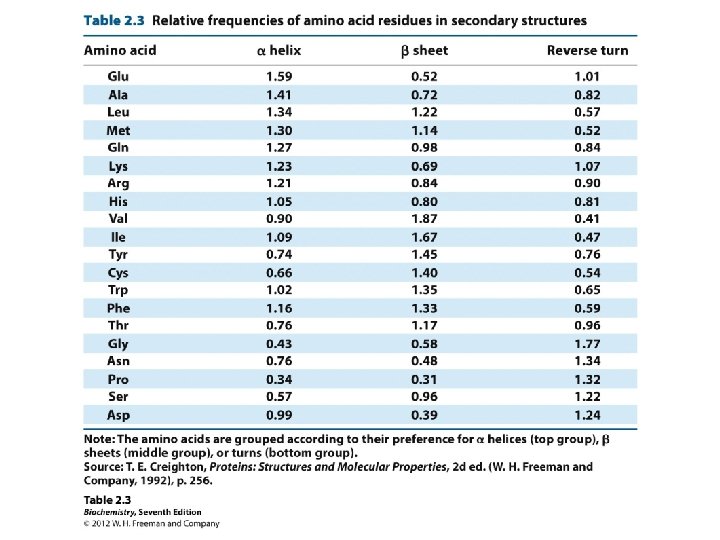
Certain amino acid R groups destabilize a-helices • valine (V), threonine (T), and isoleucine (I) with bulky groups at the β-carbon tend to destabilize α-helices because of steric clashes • serine (S), aspartate (D), and asparagine (N) side chains contain hydrogen-bond donors or acceptors that are close to the main chain, where they can compete for main-chain NH and CO groups • proline (P) also is a helix breaker because the ring structure of its side chain blocks the NH group and does not allow the φ value required to fit into an α-helix.

The amino acid R groups project out from an a-helix

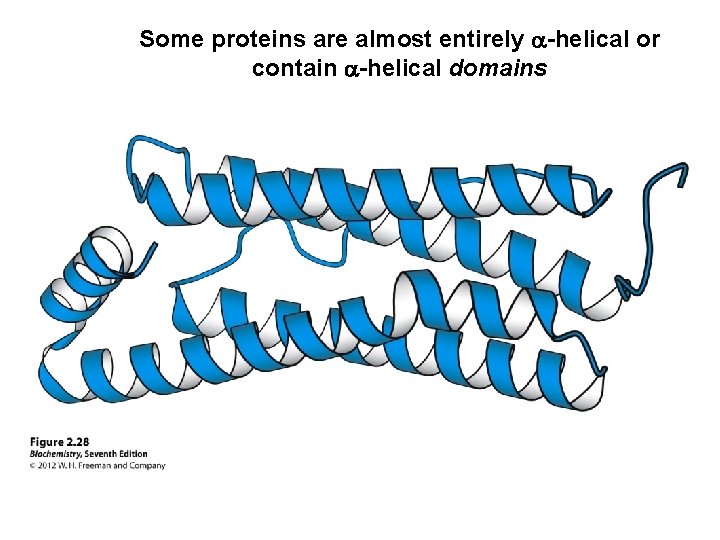
Some proteins are almost entirely a-helical or contain a-helical domains

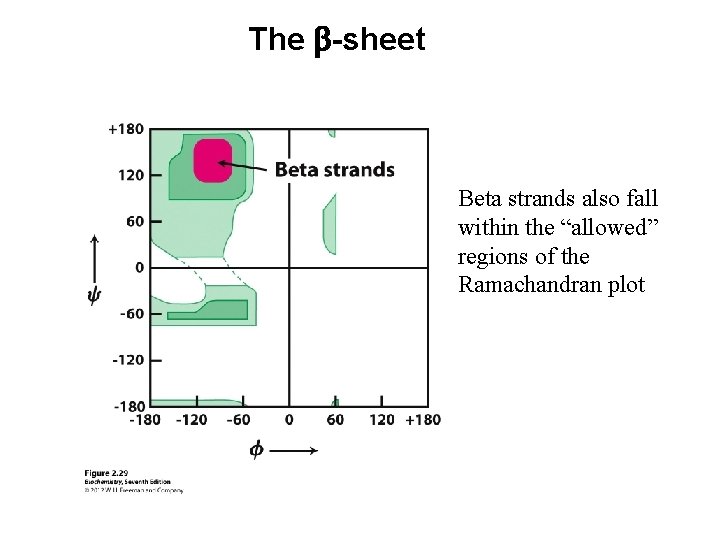
The b-sheet Beta strands also fall within the “allowed” regions of the Ramachandran plot

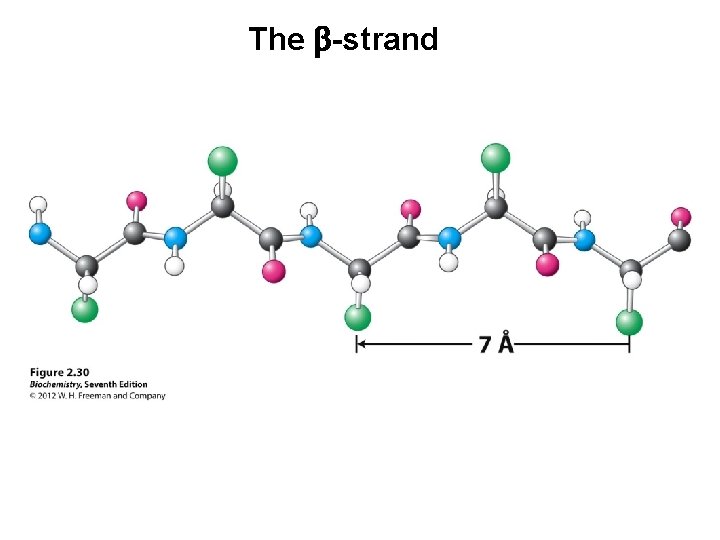
The b-strand

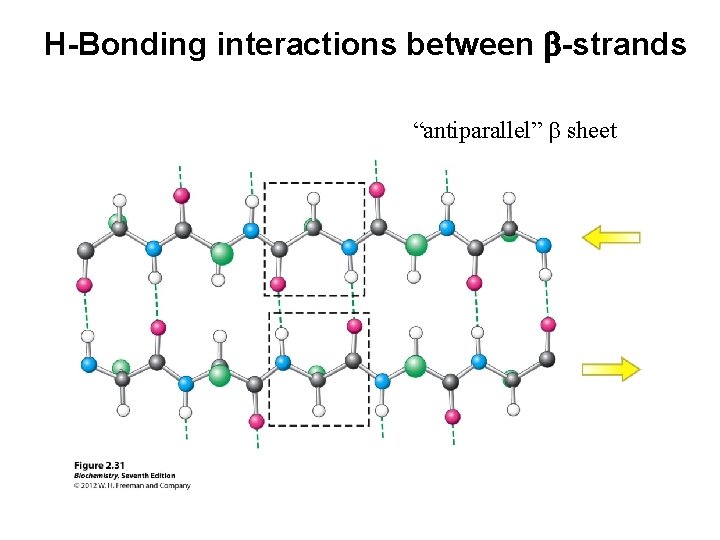
H-Bonding interactions between b-strands “antiparallel” b sheet

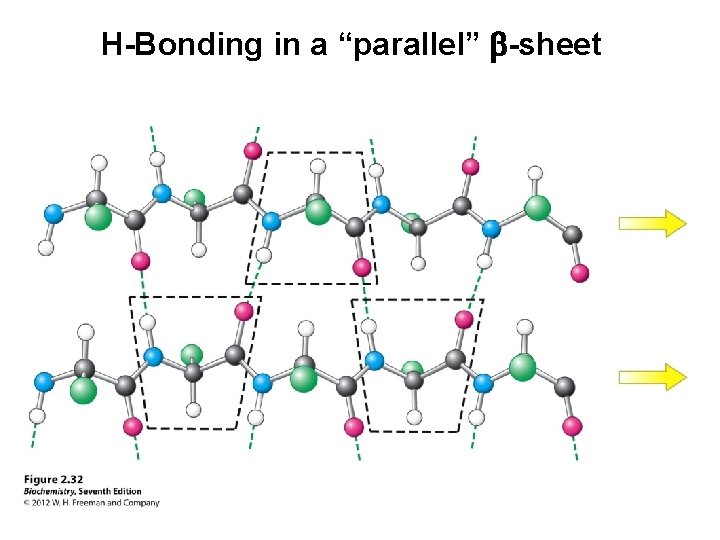
H-Bonding in a “parallel” b-sheet

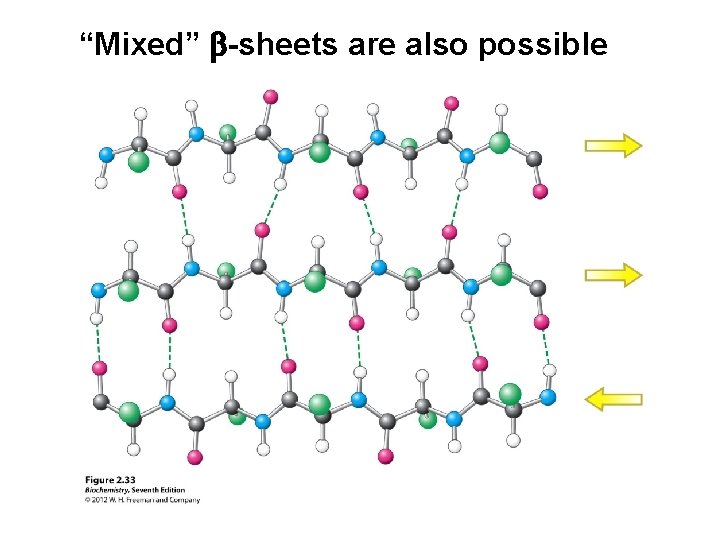
“Mixed” b-sheets are also possible

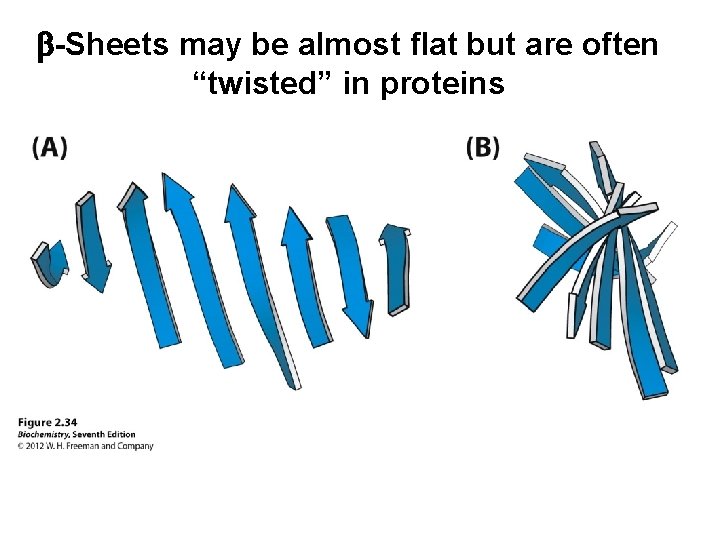
b-Sheets may be almost flat but are often “twisted” in proteins

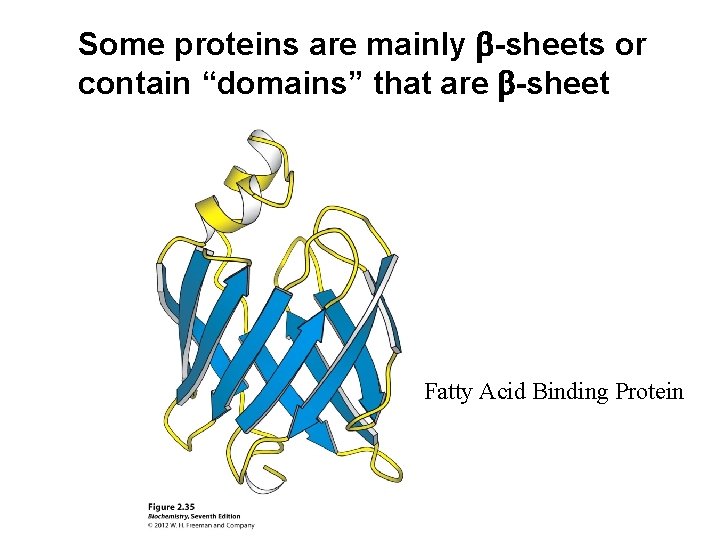
Some proteins are mainly b-sheets or contain “domains” that are b-sheet Fatty Acid Binding Protein

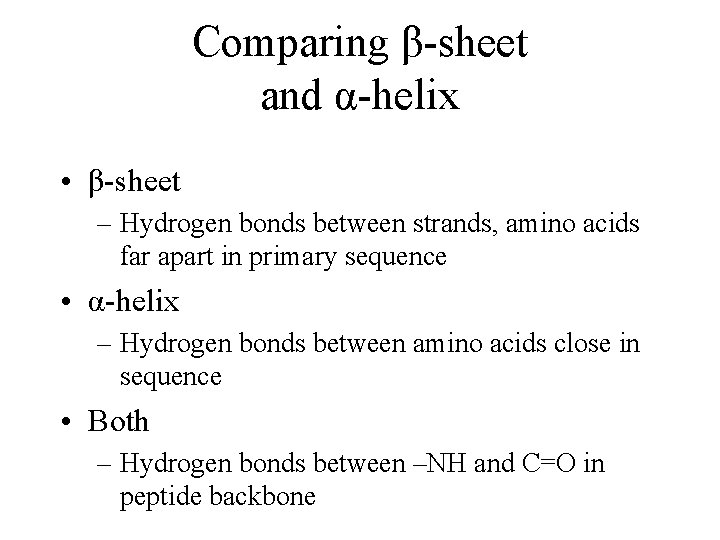
Comparing β-sheet and α-helix • β-sheet – Hydrogen bonds between strands, amino acids far apart in primary sequence • α-helix – Hydrogen bonds between amino acids close in sequence • Both – Hydrogen bonds between –NH and C=O in peptide backbone

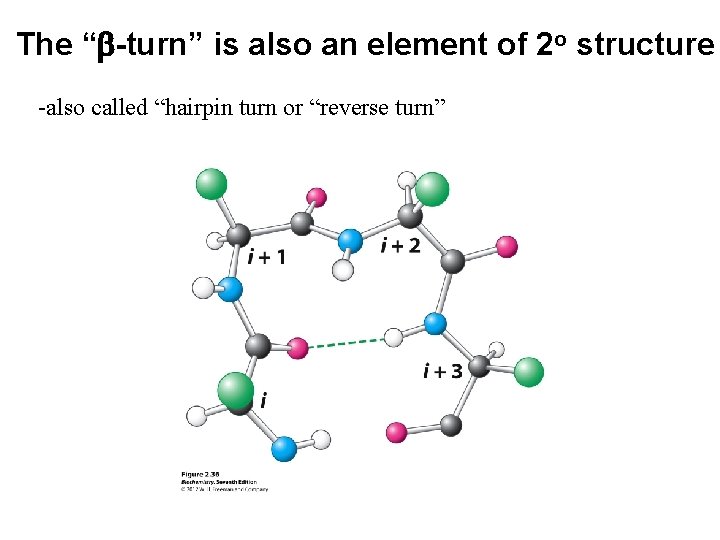
The “b-turn” is also an element of 2 o structure -also called “hairpin turn or “reverse turn”


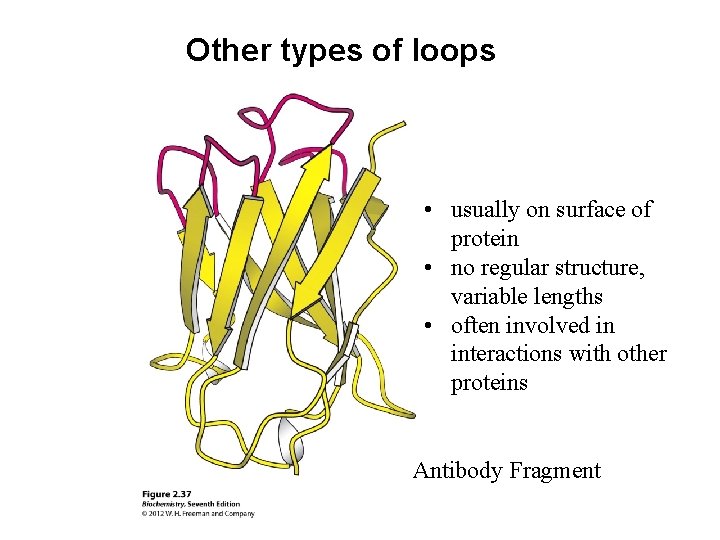
Other types of loops • usually on surface of protein • no regular structure, variable lengths • often involved in interactions with other proteins Antibody Fragment

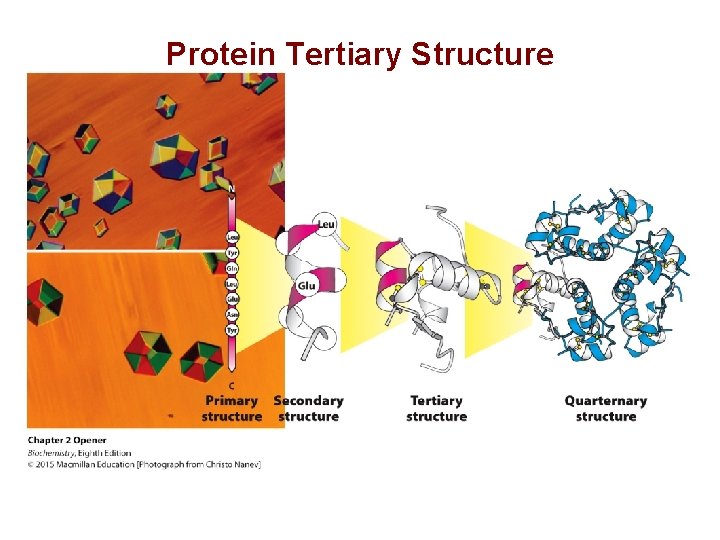
Protein Tertiary Structure

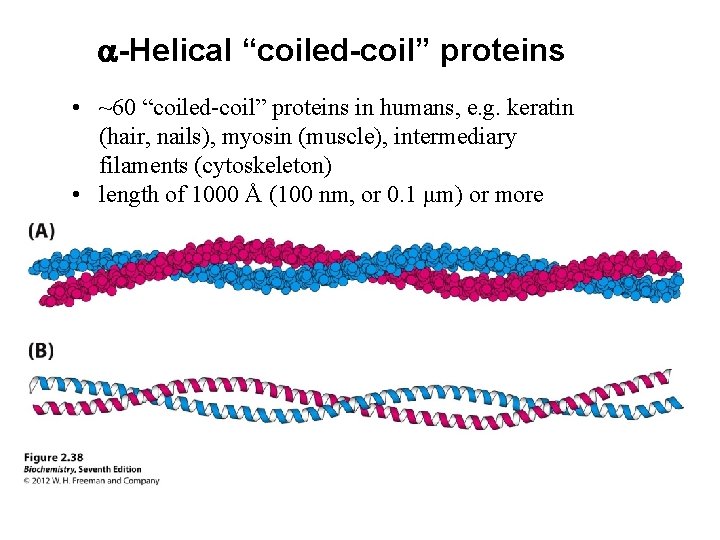
a-Helical “coiled-coil” proteins • ~60 “coiled-coil” proteins in humans, e. g. keratin (hair, nails), myosin (muscle), intermediary filaments (cytoskeleton) • length of 1000 Å (100 nm, or 0. 1 μm) or more

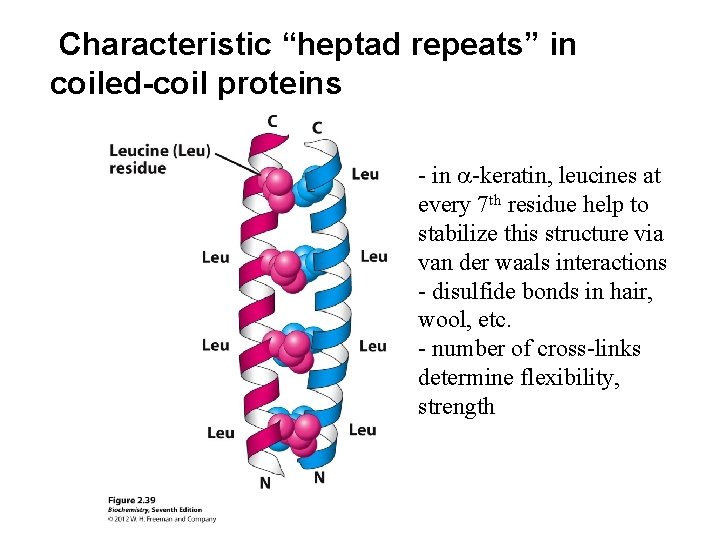
Characteristic “heptad repeats” in coiled-coil proteins - in a-keratin, leucines at every 7 th residue help to stabilize this structure via van der waals interactions - disulfide bonds in hair, wool, etc. - number of cross-links determine flexibility, strength

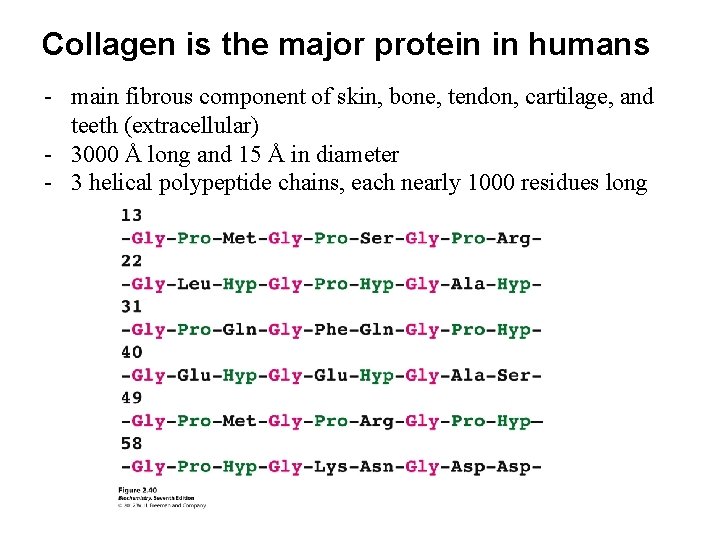
Collagen is the major protein in humans - main fibrous component of skin, bone, tendon, cartilage, and teeth (extracellular) - 3000 Å long and 15 Å in diameter - 3 helical polypeptide chains, each nearly 1000 residues long

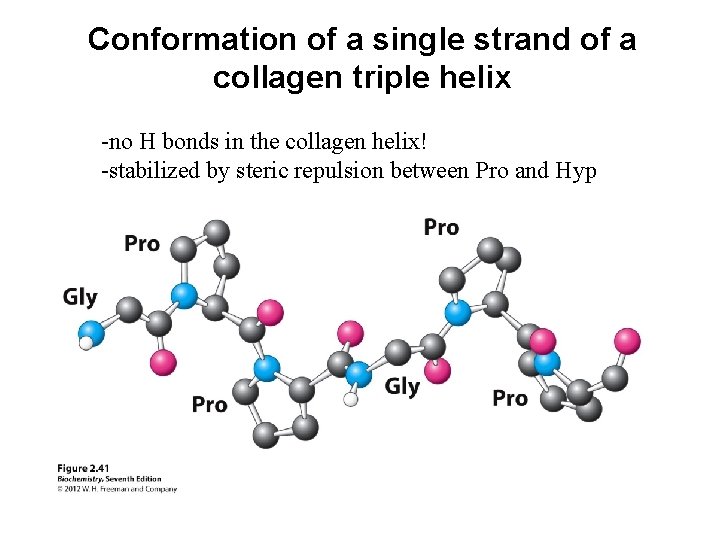
Conformation of a single strand of a collagen triple helix -no H bonds in the collagen helix! -stabilized by steric repulsion between Pro and Hyp

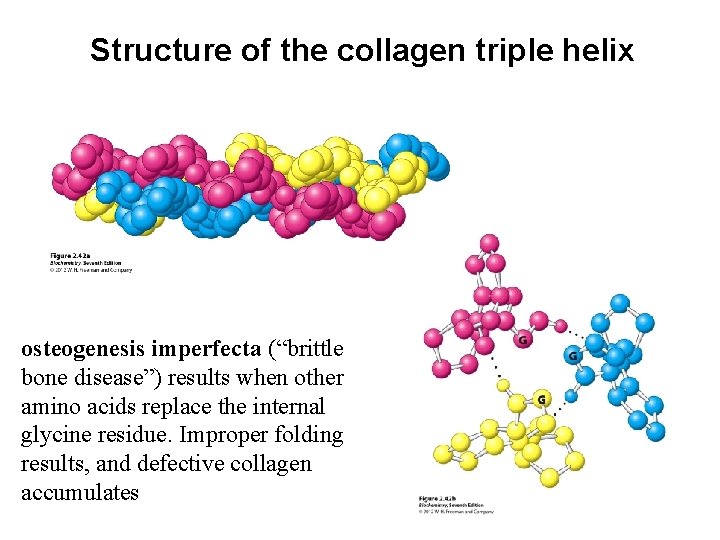
Structure of the collagen triple helix osteogenesis imperfecta (“brittle bone disease”) results when other amino acids replace the internal glycine residue. Improper folding results, and defective collagen accumulates

Globular, water soluble proteins

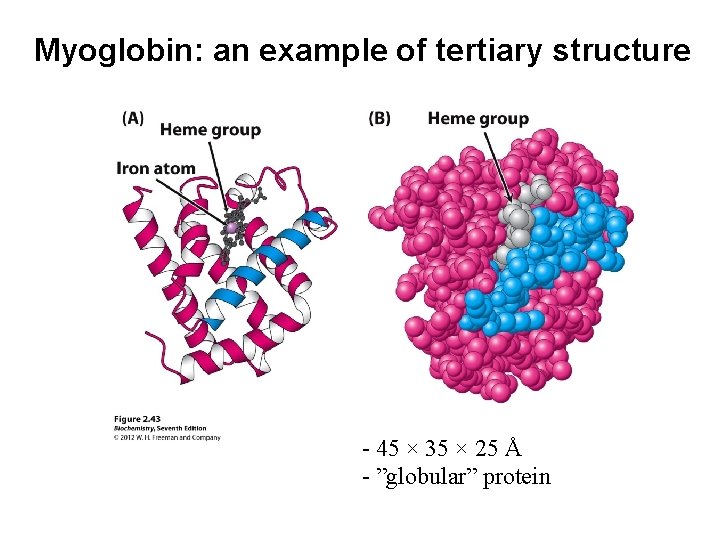
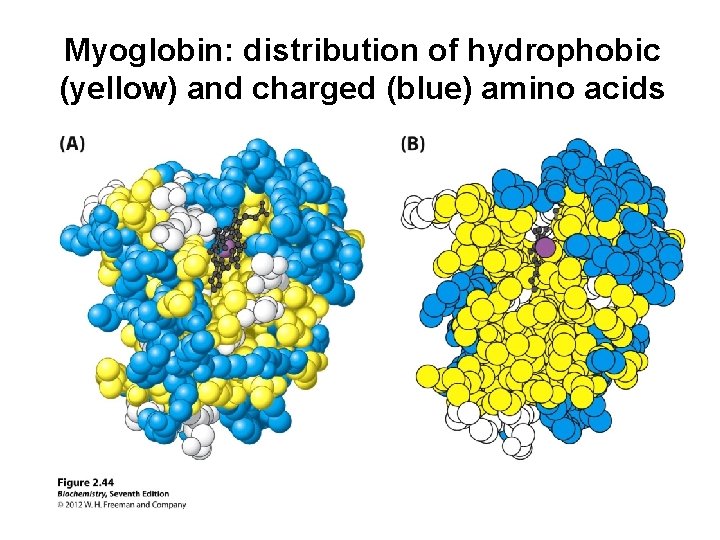
Myoglobin: an example of tertiary structure - 45 × 35 × 25 Å - ”globular” protein


Myoglobin: distribution of hydrophobic (yellow) and charged (blue) amino acids

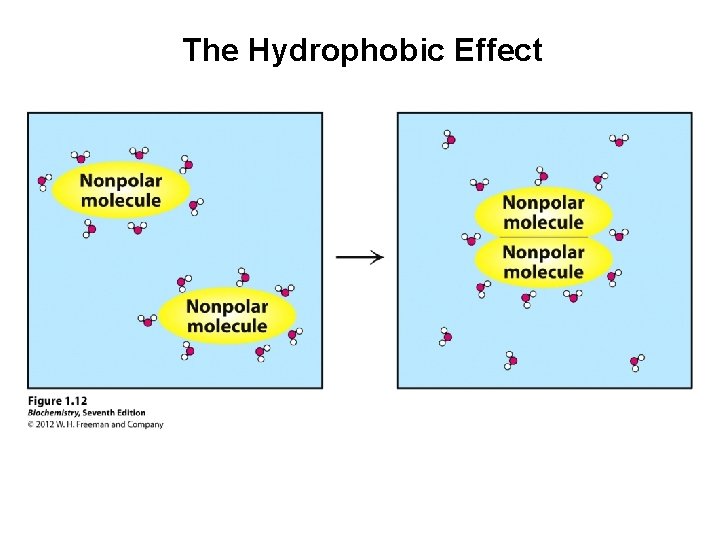
The Hydrophobic Effect

Gibb’s free energy • ΔG = ΔH –TΔS • If ΔG < 0, reaction is favourable (spontaneous)

Amino acids and polypeptides in a hydrophobic environment • polypeptide backbone in a hydrophobic environment is stabilized by pairing all the NH and CO groups by Hbonding: α helix or β sheet. • α helix and β sheet can have hydrophobic and hydrophilic “side” • van der Waals interactions between adjacent hydrocarbon side chains also contribute to protein stability • with 20 amino acids of different sizes and shapes interior of a protein can be tightly packed to maximize van der Waals interactions • ionic, H-bonding interactions between side chains also

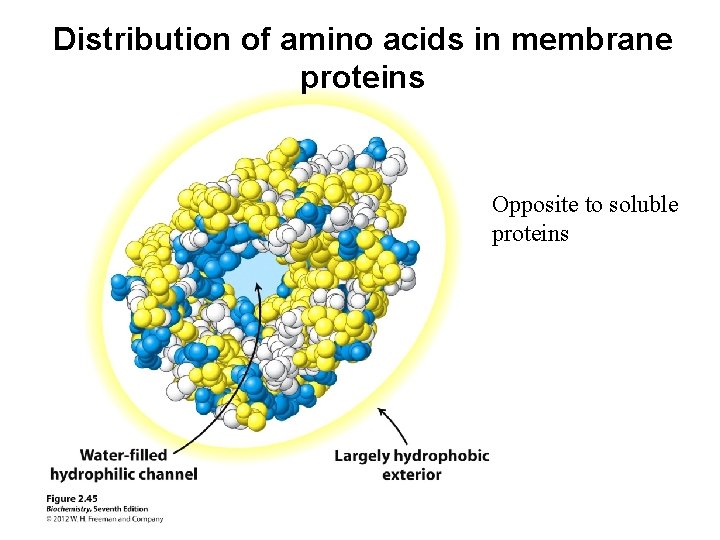
Distribution of amino acids in membrane proteins Opposite to soluble proteins

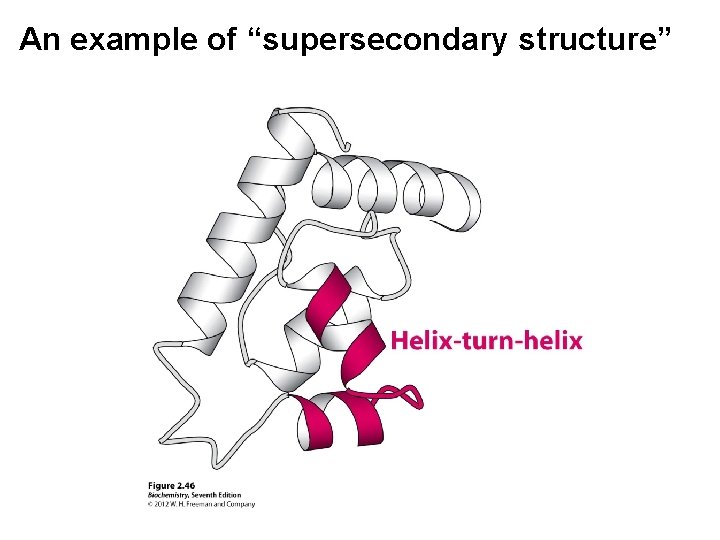
An example of “supersecondary structure”

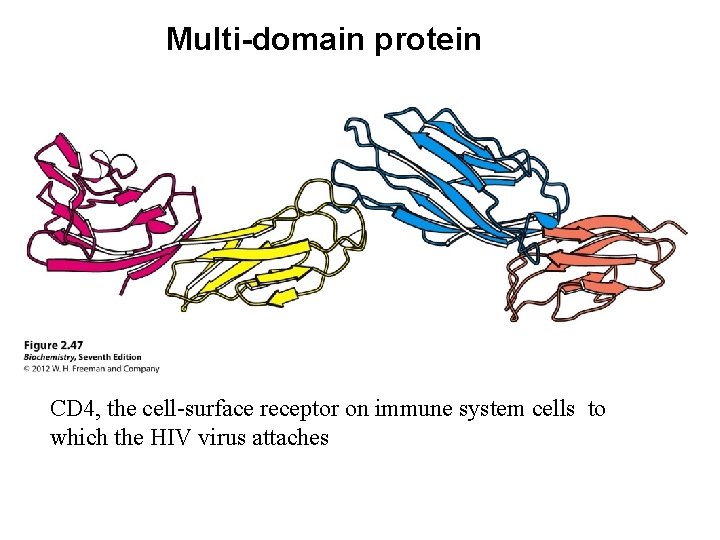
Multi-domain protein CD 4, the cell-surface receptor on immune system cells to which the HIV virus attaches