Protein Structure Folding 9 25 2008 Secondary Structure



- Slides: 38

Protein Structure & Folding (9 / 25 / 2008) • Secondary Structure • Tertiary Structure • Quaternary Structure and Symmetry • Protein Folding

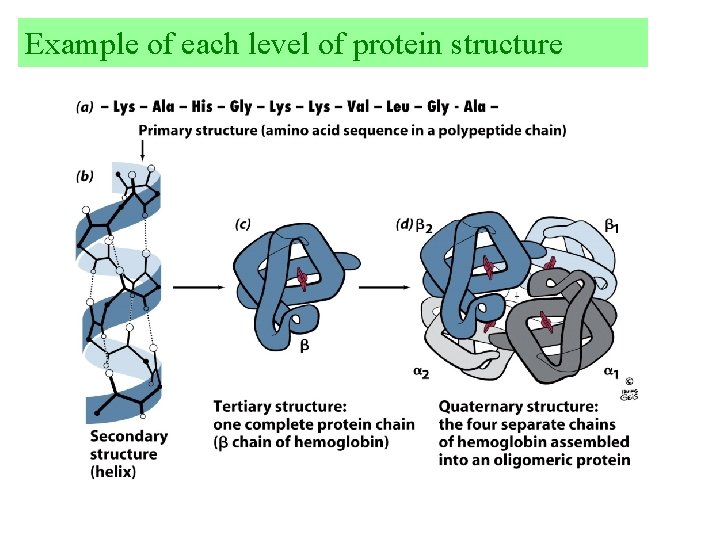
Example of each level of protein structure

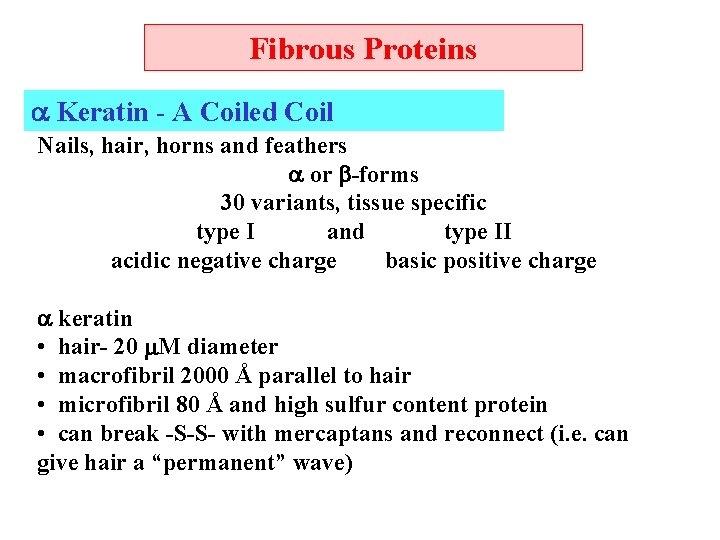
Fibrous Proteins Keratin - A Coiled Coil Nails, hair, horns and feathers or -forms 30 variants, tissue specific type I and type II acidic negative charge basic positive charge keratin • hair- 20 M diameter • macrofibril 2000 Å parallel to hair • microfibril 80 Å and high sulfur content protein • can break -S-S- with mercaptans and reconnect (i. e. can give hair a “permanent” wave)

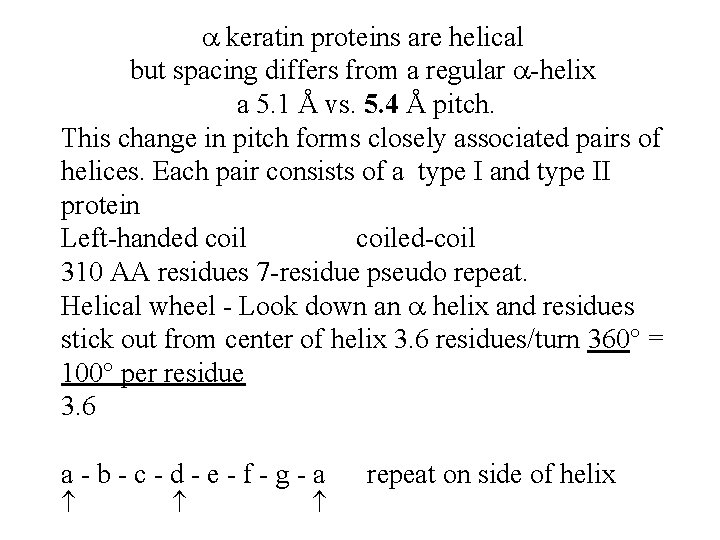
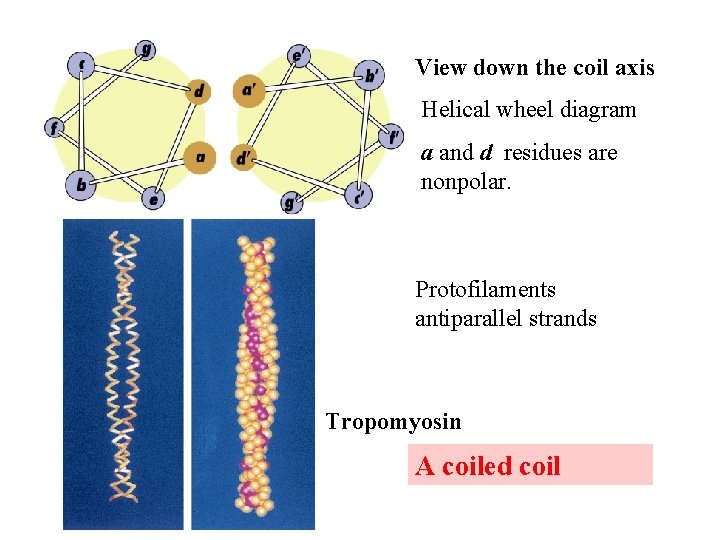
keratin proteins are helical but spacing differs from a regular -helix a 5. 1 Å vs. 5. 4 Å pitch. This change in pitch forms closely associated pairs of helices. Each pair consists of a type I and type II protein Left-handed coiled-coil 310 AA residues 7 -residue pseudo repeat. Helical wheel - Look down an helix and residues stick out from center of helix 3. 6 residues/turn 360 = 100 per residue 3. 6 a-b-c-d-e-f-g-a repeat on side of helix

View down the coil axis Helical wheel diagram a and d residues are nonpolar. Protofilaments antiparallel strands Tropomyosin A coiled coil

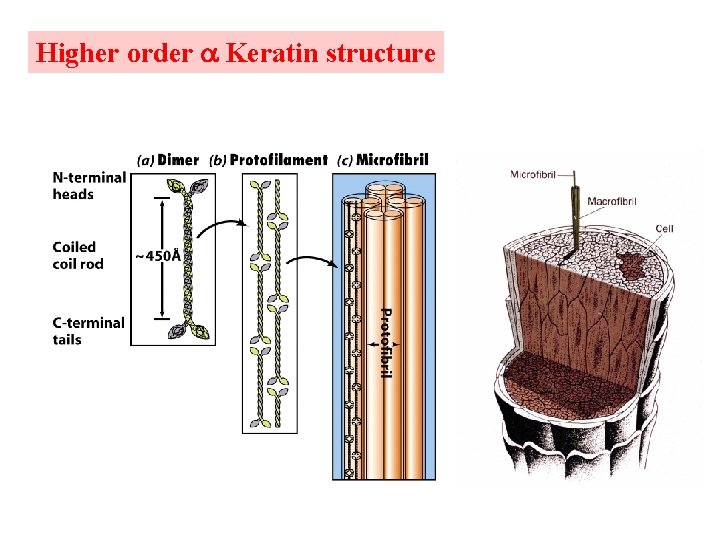
a & d are non-polar and face the same side of helix. 3. 6 residues/turn 3. 5 residues hydrophobic repeat The hydrophobic strip aligns between two helices with 18 inclination from one to another. Dimer protofilament microfibril macrofibril hair keratin rich in cys and form disulfides hard keratin cys content is high soft keratin cys content is low Perms reduce R-S--S-R bonds to 2 R-SH Curly hair has more Cys residues.

Higher order Keratin structure

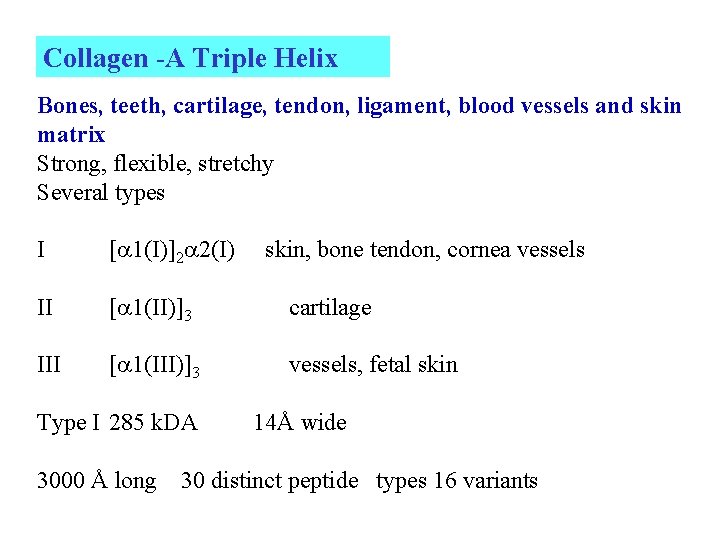
Collagen -A Triple Helix Bones, teeth, cartilage, tendon, ligament, blood vessels and skin matrix Strong, flexible, stretchy Several types I [ 1(I)]2 2(I) II [ 1(II)]3 cartilage III [ 1(III)]3 vessels, fetal skin Type I 285 k. DA 3000 Å long skin, bone tendon, cornea vessels 14Å wide 30 distinct peptide types 16 variants

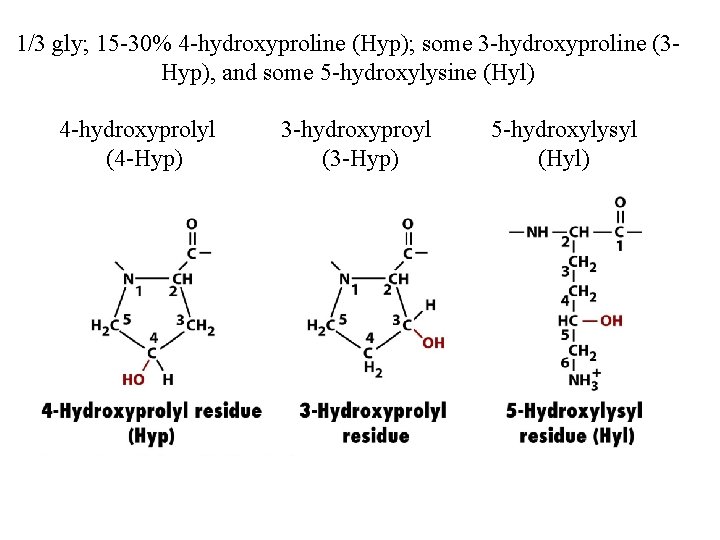
1/3 gly; 15 -30% 4 -hydroxyproline (Hyp); some 3 -hydroxyproline (3 Hyp), and some 5 -hydroxylysine (Hyl) 4 -hydroxyprolyl (4 -Hyp) 3 -hydroxyproyl (3 -Hyp) 5 -hydroxylysyl (Hyl)

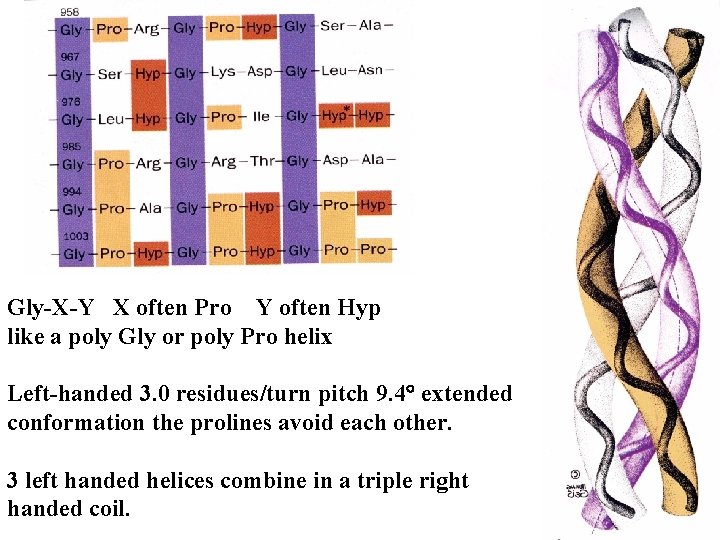
Gly-X-Y X often Pro Y often Hyp like a poly Gly or poly Pro helix Left-handed 3. 0 residues/turn pitch 9. 4 extended conformation the prolines avoid each other. 3 left handed helices combine in a triple right handed coil.

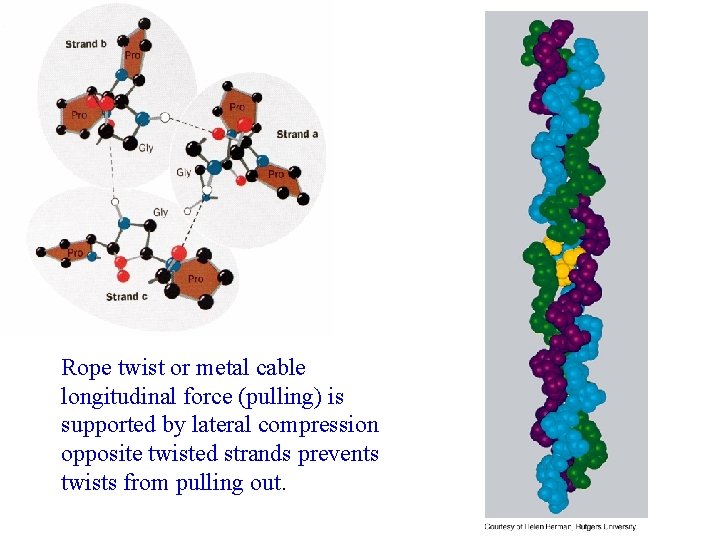
Rope twist or metal cable longitudinal force (pulling) is supported by lateral compression opposite twisted strands prevents twists from pulling out.

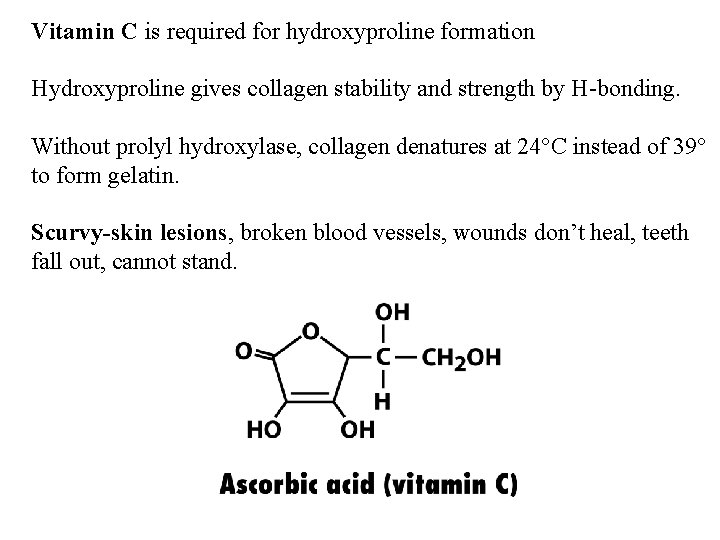
Vitamin C is required for hydroxyproline formation Hydroxyproline gives collagen stability and strength by H-bonding. Without prolyl hydroxylase, collagen denatures at 24 C instead of 39 to form gelatin. Scurvy-skin lesions, broken blood vessels, wounds don’t heal, teeth fall out, cannot stand.

Crosslinking requires lysine oxidase N C - CH 2 - NH 2 sweet pea -Aminopropionitrile, from Inhibits lysine oxidase i. e. no crosslinking several diseases: Lathyrism (abnormalities in bones, joints, etc. ) Osteogenesis imperfecta, brittle bone, A single amino acid change could be lethal Ehlers-Danlos syndromes, hyperextensible joints and skin, Indian rubberman Osteoarthritis - cartilage.

Tertiary protein structure • Describes the folding of its secondary structural elements • Determined via nuclear magnetic resonance (NMR) spectroscopy and X-ray crystallography • 3 D structures of many proteins are available at http: //www. rcsb. org/pdb The Protein Data Bank (PDB)

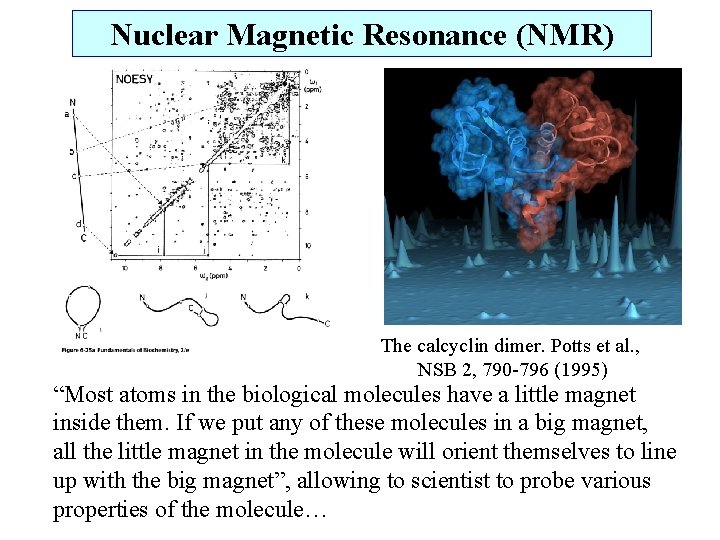
Nuclear Magnetic Resonance (NMR) The calcyclin dimer. Potts et al. , NSB 2, 790 -796 (1995) “Most atoms in the biological molecules have a little magnet inside them. If we put any of these molecules in a big magnet, all the little magnet in the molecule will orient themselves to line up with the big magnet”, allowing to scientist to probe various properties of the molecule…

NMR at the UH • The UH Keck/IMD NMR Center has the first 800 MHz NMR spectrometer installed within Texas. • The latest facility enhancement, in January 2006, is the installation of a Bruker 5 mm TXI Cryo. Probe for the 800 MHz instrument. UH 800 MHz NMR Spectrometer – This NMR probehead is cooled by cyrogenic helium gas to reduce thermal noise and improve the signal to noise ratio by as much as three times a conventional probe.

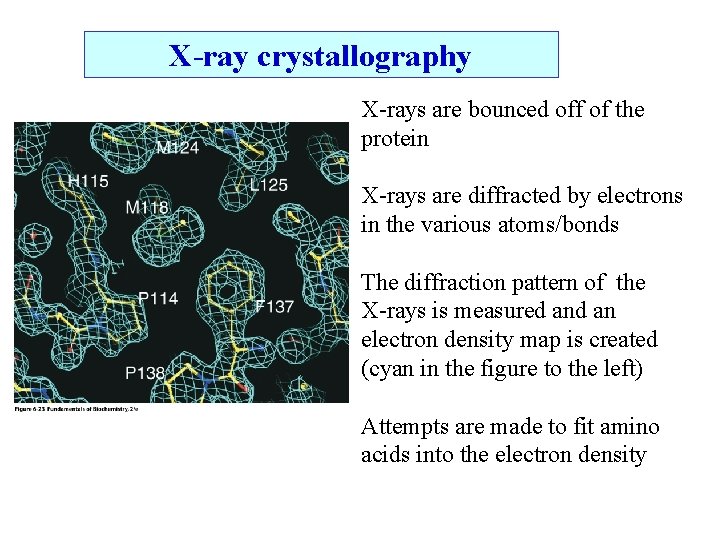
X-ray crystallography X-rays are bounced off of the protein X-rays are diffracted by electrons in the various atoms/bonds The diffraction pattern of the X-rays is measured an electron density map is created (cyan in the figure to the left) Attempts are made to fit amino acids into the electron density

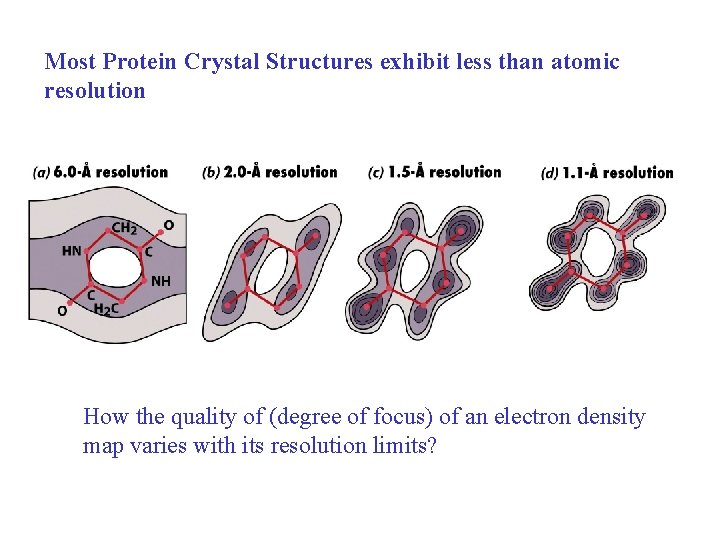
Most Protein Crystal Structures exhibit less than atomic resolution How the quality of (degree of focus) of an electron density map varies with its resolution limits?

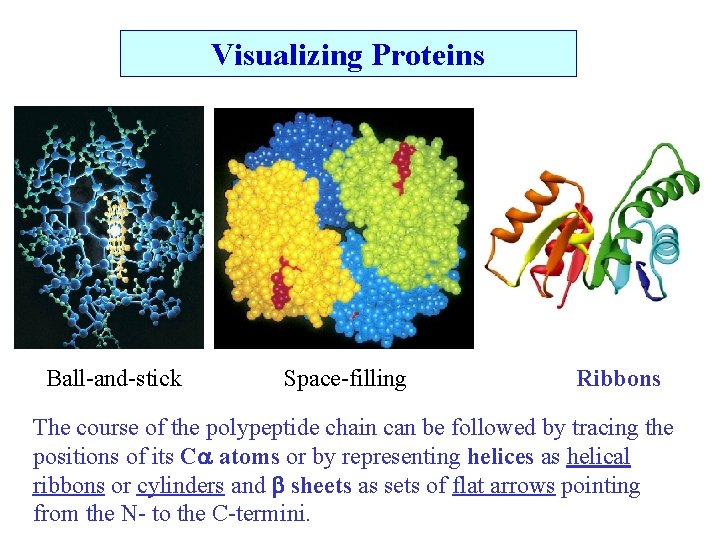
Visualizing Proteins Ball-and-stick Space-filling Ribbons The course of the polypeptide chain can be followed by tracing the positions of its C atoms or by representing helices as helical ribbons or cylinders and sheets as sets of flat arrows pointing from the N- to the C-termini.

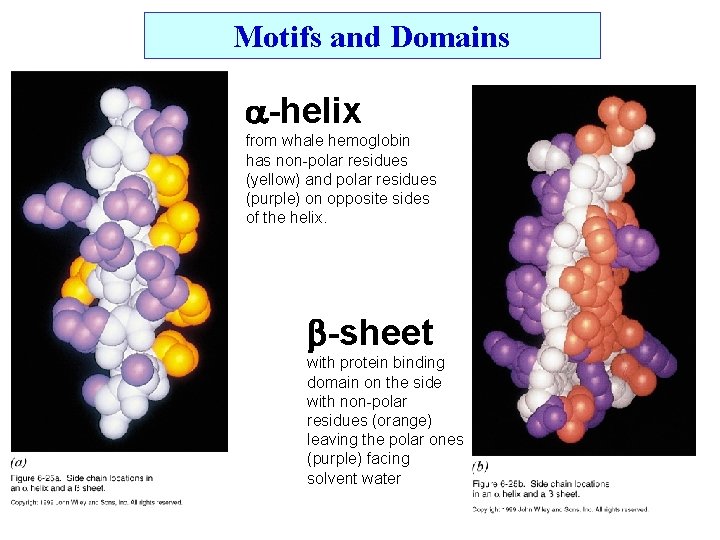
Motifs and Domains -helix from whale hemoglobin has non-polar residues (yellow) and polar residues (purple) on opposite sides of the helix. -sheet with protein binding domain on the side with non-polar residues (orange) leaving the polar ones (purple) facing solvent water

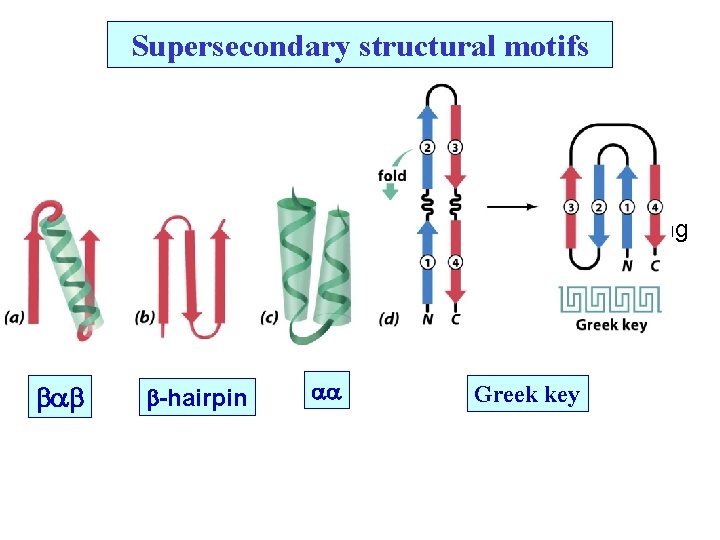
Supersecondary structural motifs Nucleotide binding Rossman Fold b b b Most common Common -hairpin Greek key

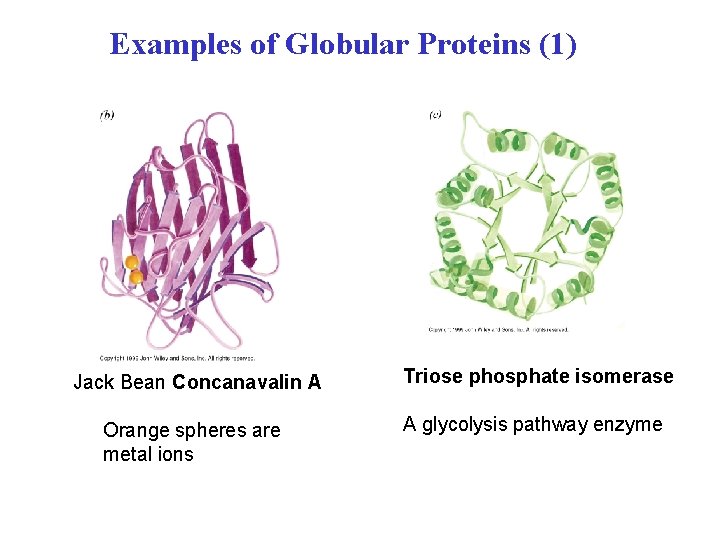
Examples of Globular Proteins (1) Jack Bean Concanavalin A Triose phosphate isomerase Orange spheres are metal ions A glycolysis pathway enzyme

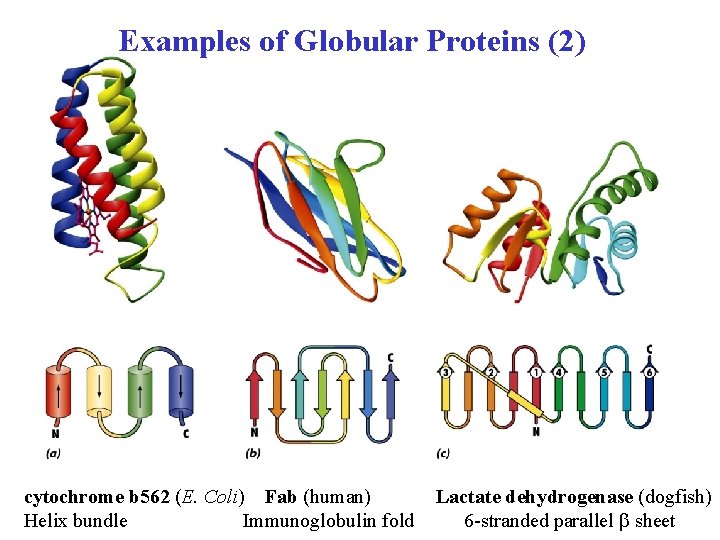
Examples of Globular Proteins (2) cytochrome b 562 (E. Coli) Fab (human) Helix bundle Immunoglobulin fold Lactate dehydrogenase (dogfish) 6 -stranded parallel b sheet

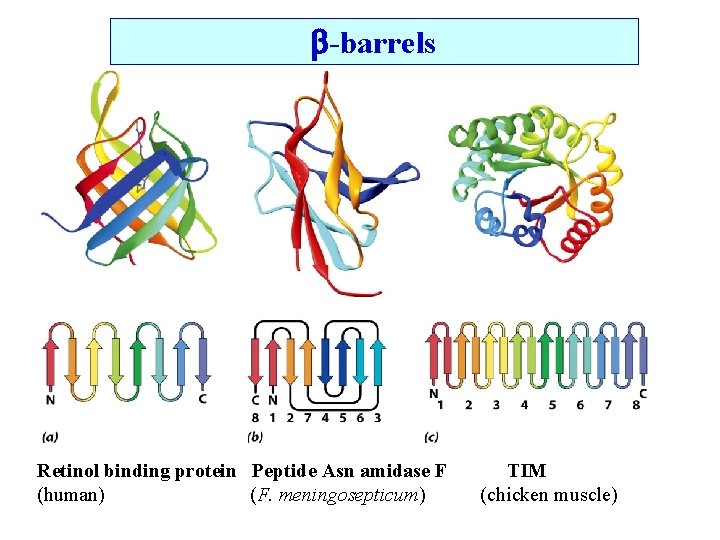
-barrels Retinol binding protein Peptide Asn amidase F (human) (F. meningosepticum) TIM (chicken muscle)

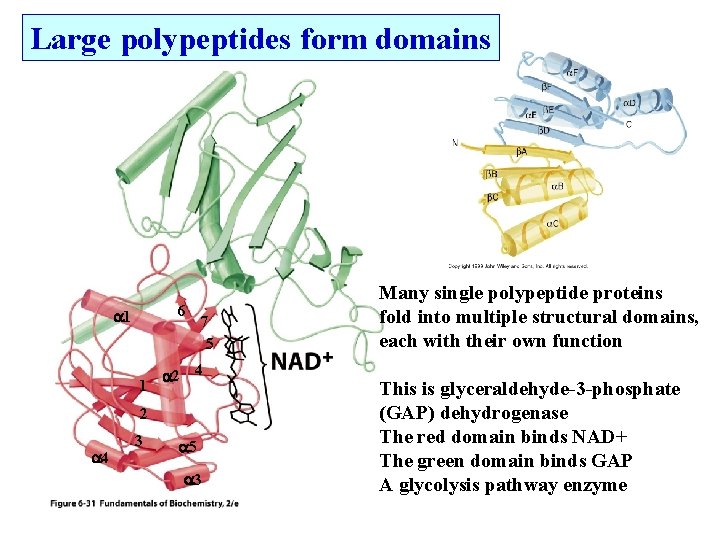
Large polypeptides form domains 6 1 1 7 5 2 4 3 5 3 Many single polypeptide proteins fold into multiple structural domains, each with their own function This is glyceraldehyde-3 -phosphate (GAP) dehydrogenase The red domain binds NAD+ The green domain binds GAP A glycolysis pathway enzyme

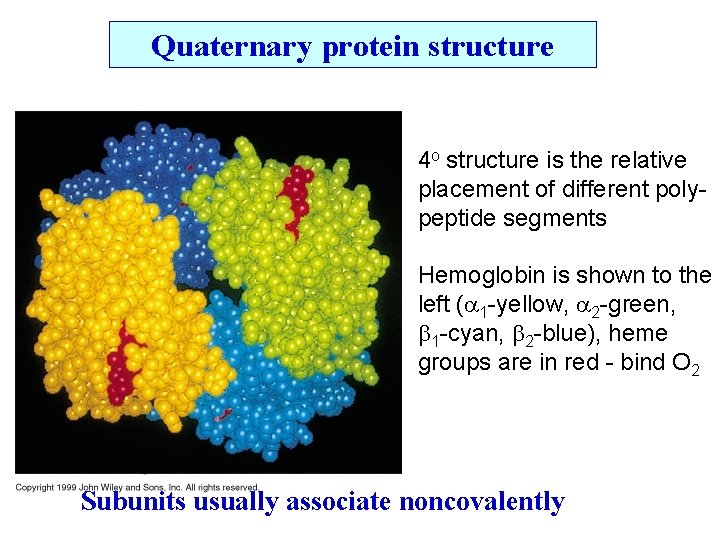
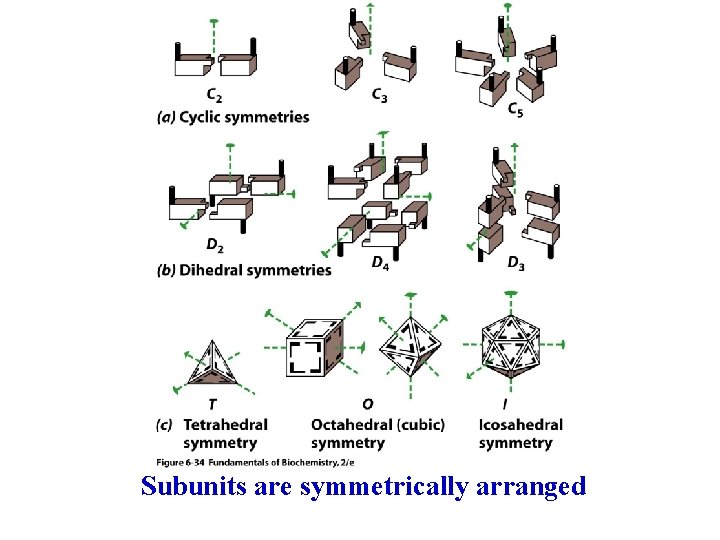
Quaternary protein structure 4 o structure is the relative placement of different polypeptide segments Hemoglobin is shown to the left ( 1 -yellow, 2 -green, b 1 -cyan, b 2 -blue), heme groups are in red - bind O 2 Subunits usually associate noncovalently

Subunits are symmetrically arranged

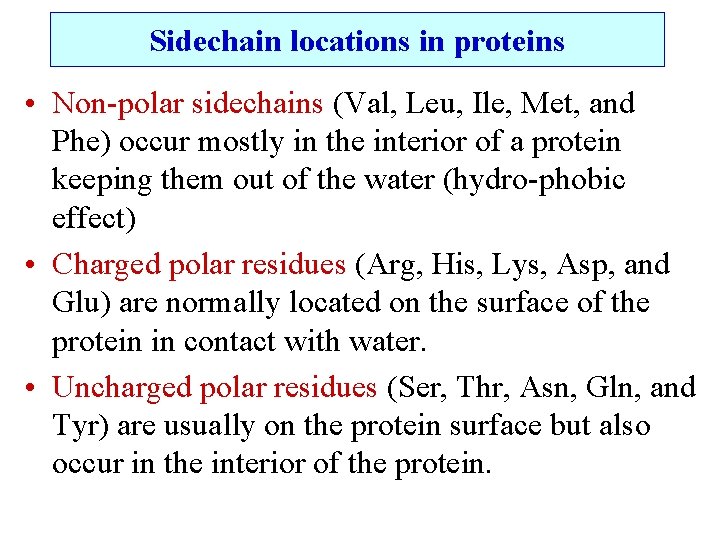
Sidechain locations in proteins • Non-polar sidechains (Val, Leu, Ile, Met, and Phe) occur mostly in the interior of a protein keeping them out of the water (hydro-phobic effect) • Charged polar residues (Arg, His, Lys, Asp, and Glu) are normally located on the surface of the protein in contact with water. • Uncharged polar residues (Ser, Thr, Asn, Gln, and Tyr) are usually on the protein surface but also occur in the interior of the protein.

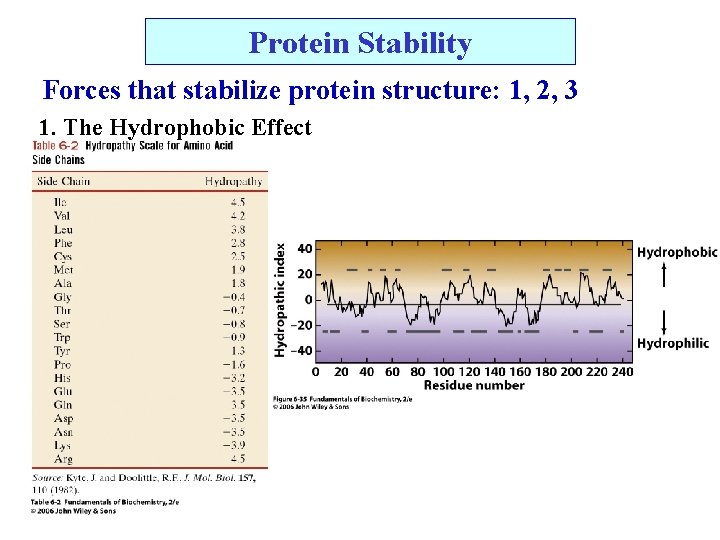
Protein Stability Forces that stabilize protein structure: 1, 2, 3 1. The Hydrophobic Effect

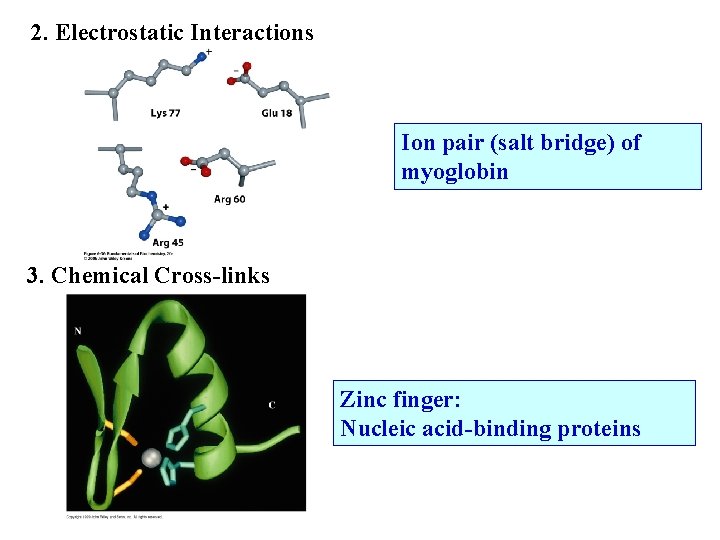
2. Electrostatic Interactions Ion pair (salt bridge) of myoglobin 3. Chemical Cross-links Zinc finger: Nucleic acid-binding proteins

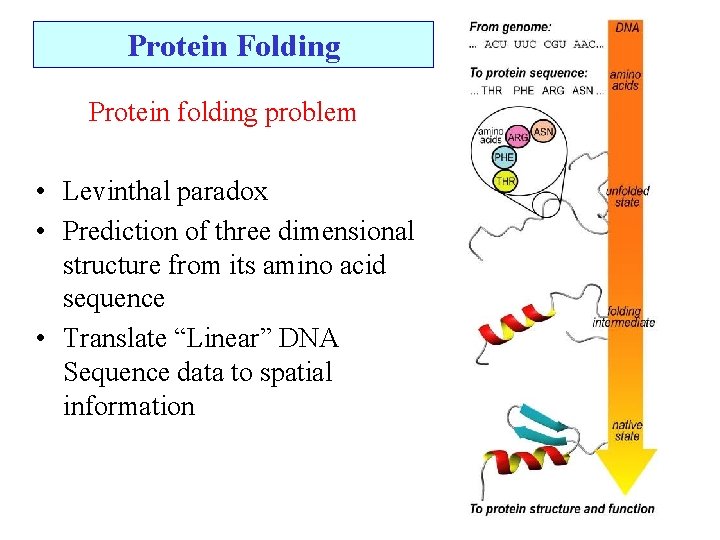
Protein Folding Protein folding problem • Levinthal paradox • Prediction of three dimensional structure from its amino acid sequence • Translate “Linear” DNA Sequence data to spatial information

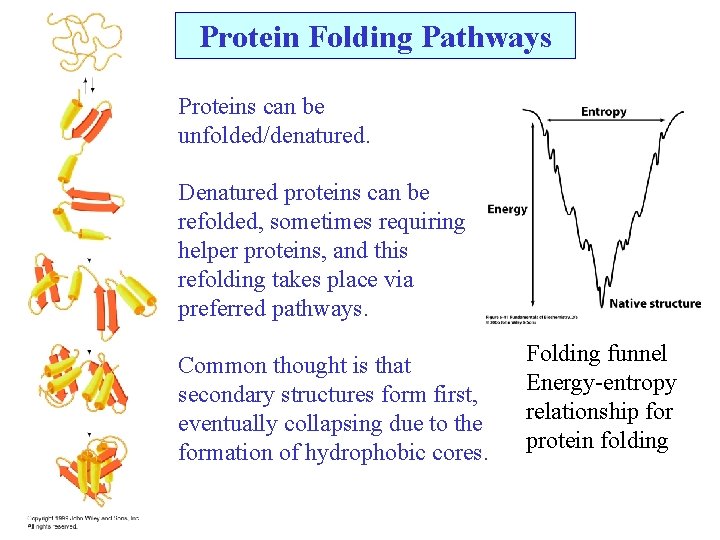
Protein Folding Pathways Proteins can be unfolded/denatured. Denatured proteins can be refolded, sometimes requiring helper proteins, and this refolding takes place via preferred pathways. Common thought is that secondary structures form first, eventually collapsing due to the formation of hydrophobic cores. Folding funnel Energy-entropy relationship for protein folding

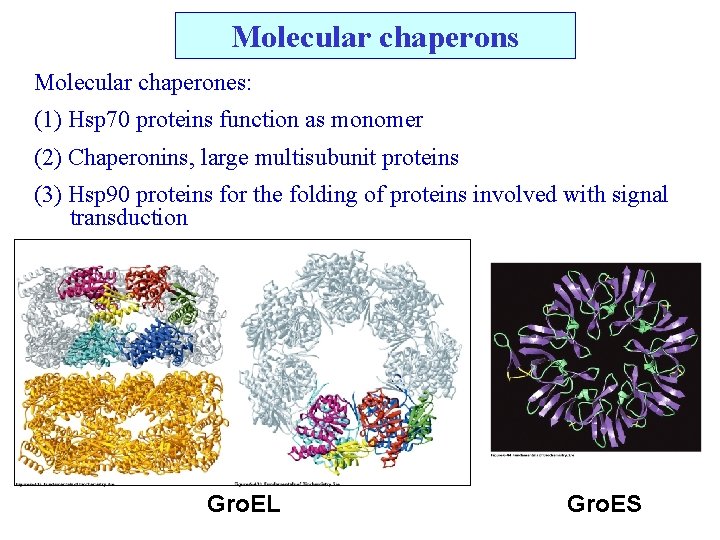
Molecular chaperons Molecular chaperones: (1) Hsp 70 proteins function as monomer (2) Chaperonins, large multisubunit proteins (3) Hsp 90 proteins for the folding of proteins involved with signal transduction Gro. EL Gro. ES

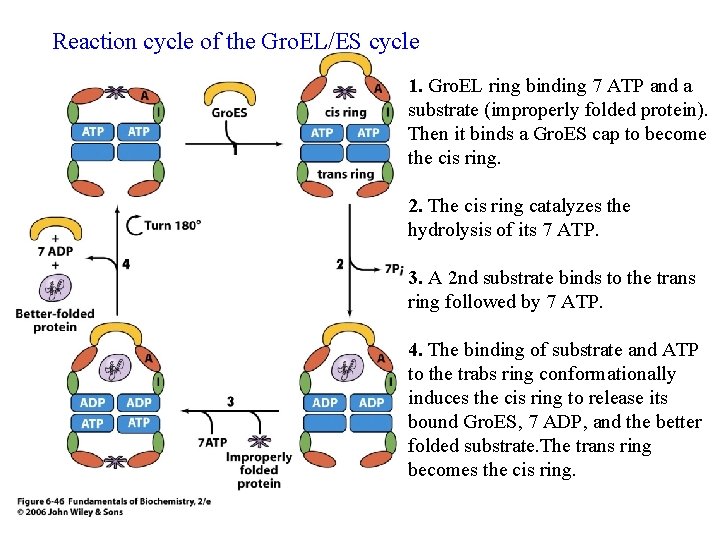
Reaction cycle of the Gro. EL/ES cycle 1. Gro. EL ring binding 7 ATP and a substrate (improperly folded protein). Then it binds a Gro. ES cap to become the cis ring. 2. The cis ring catalyzes the hydrolysis of its 7 ATP. 3. A 2 nd substrate binds to the trans ring followed by 7 ATP. 4. The binding of substrate and ATP to the trabs ring conformationally induces the cis ring to release its bound Gro. ES, 7 ADP, and the better folded substrate. The trans ring becomes the cis ring.

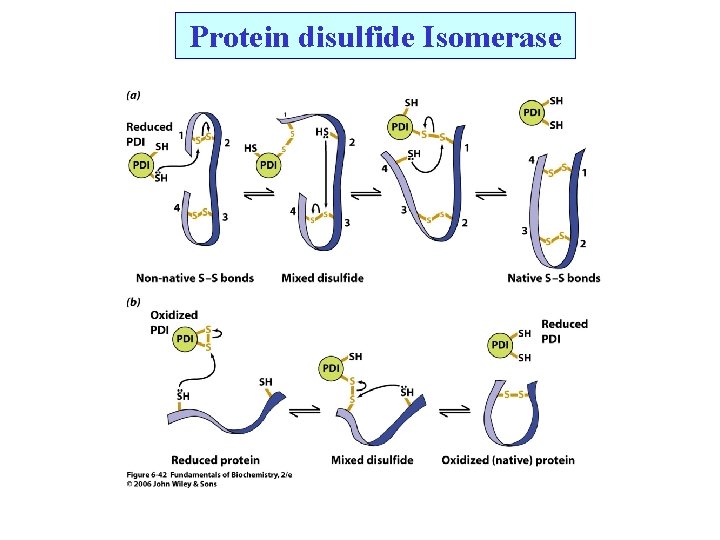
Protein disulfide Isomerase

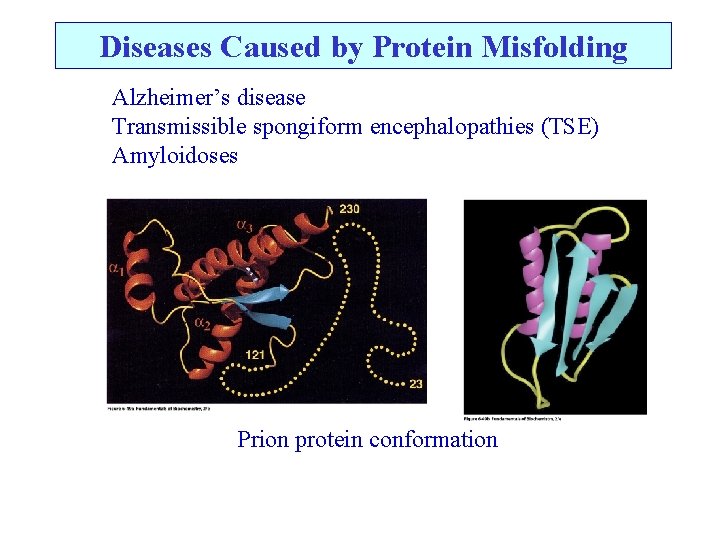
Diseases Caused by Protein Misfolding Alzheimer’s disease Transmissible spongiform encephalopathies (TSE) Amyloidoses Prion protein conformation

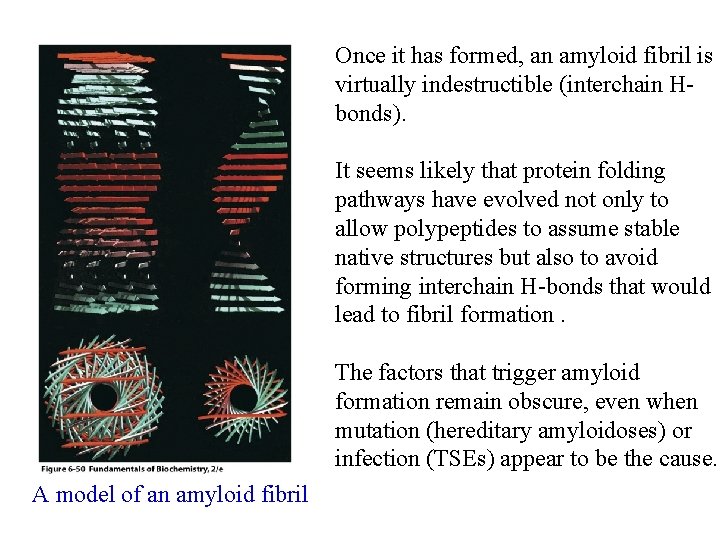
Once it has formed, an amyloid fibril is virtually indestructible (interchain Hbonds). It seems likely that protein folding pathways have evolved not only to allow polypeptides to assume stable native structures but also to avoid forming interchain H-bonds that would lead to fibril formation. The factors that trigger amyloid formation remain obscure, even when mutation (hereditary amyloidoses) or infection (TSEs) appear to be the cause. A model of an amyloid fibril

Lecture 11 Tuesday 9/30/08 Protein Structure and Purification