Protein Purification and Expression MCB 130 L Lecture
Protein Purification and Expression MCB 130 L, Lecture 2

Review of DNA Lab l l l PCR Restriction Digests Agarose Gel Electrophoresis
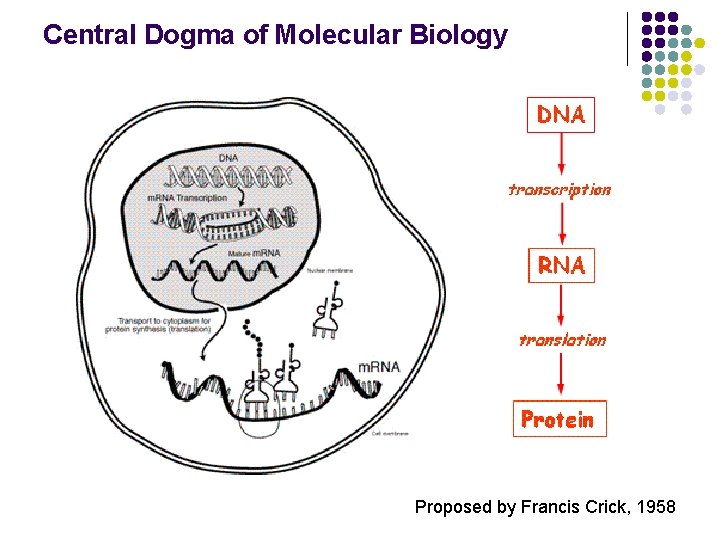
Central Dogma of Molecular Biology Proposed by Francis Crick, 1958

Why purify a protein? l l To study its function To analyze its physical properties To determine its sequence For industrial or therapeutic applications
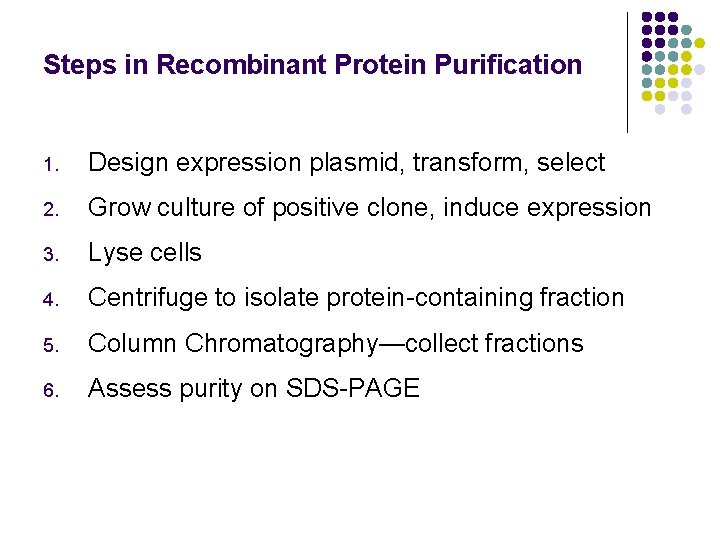
Steps in Recombinant Protein Purification 1. Design expression plasmid, transform, select 2. Grow culture of positive clone, induce expression 3. Lyse cells 4. Centrifuge to isolate protein-containing fraction 5. Column Chromatography—collect fractions 6. Assess purity on SDS-PAGE
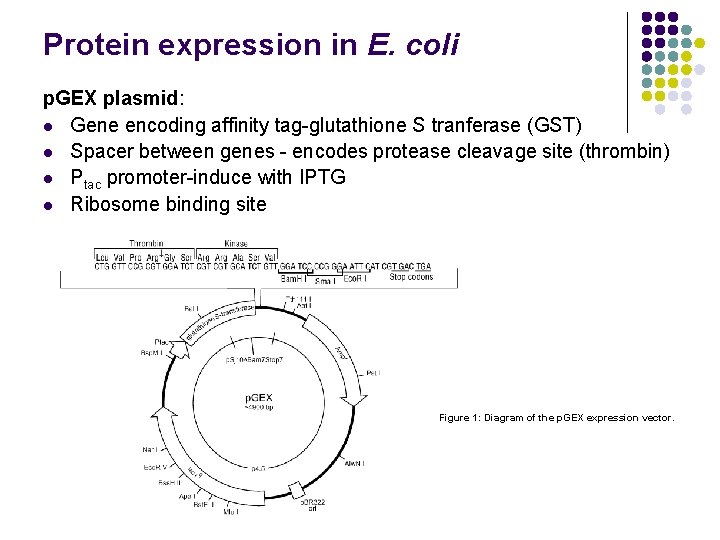
Protein expression in E. coli p. GEX plasmid: l Gene encoding affinity tag-glutathione S tranferase (GST) l Spacer between genes - encodes protease cleavage site (thrombin) l Ptac promoter-induce with IPTG l Ribosome binding site Figure 1: Diagram of the p. GEX expression vector.
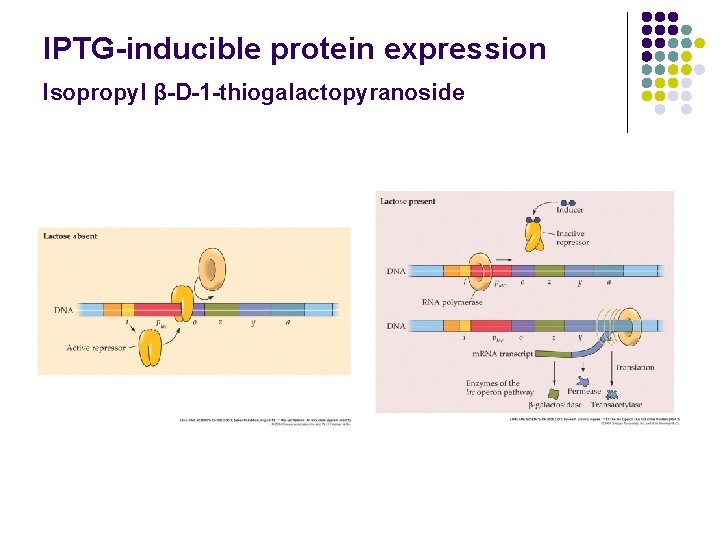
IPTG-inducible protein expression Isopropyl β-D-1 -thiogalactopyranoside
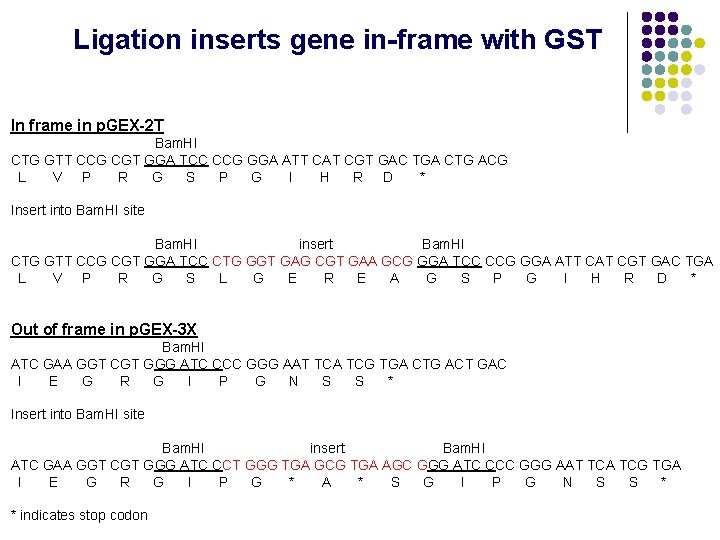
Ligation inserts gene in-frame with GST In frame in p. GEX-2 T Bam. HI CTG GTT CCG CGT GGA TCC CCG GGA ATT CAT CGT GAC TGA CTG ACG L V P R G S P G I H R D * Insert into Bam. HI site Bam. HI insert Bam. HI CTG GTT CCG CGT GGA TCC CTG GGT GAG CGT GAA GCG GGA TCC CCG GGA ATT CAT CGT GAC TGA L V P R G S L G E R E A G S P G I H R D * Out of frame in p. GEX-3 X Bam. HI ATC GAA GGT CGT GGG ATC CCC GGG AAT TCA TCG TGA CTG ACT GAC I E G R G I P G N S S * Insert into Bam. HI site Bam. HI insert Bam. HI ATC GAA GGT CGT GGG ATC CCT GGG TGA GCG TGA AGC GGG ATC CCC GGG AAT TCA TCG TGA I E G R G I P G * A * S G I P G N S S * * indicates stop codon

Cell lysis: rupture cell wall / plasma membrane, --> release contents (organelles, proteins…) 1. Physical means 2. Sonication 3. Osmotic shock
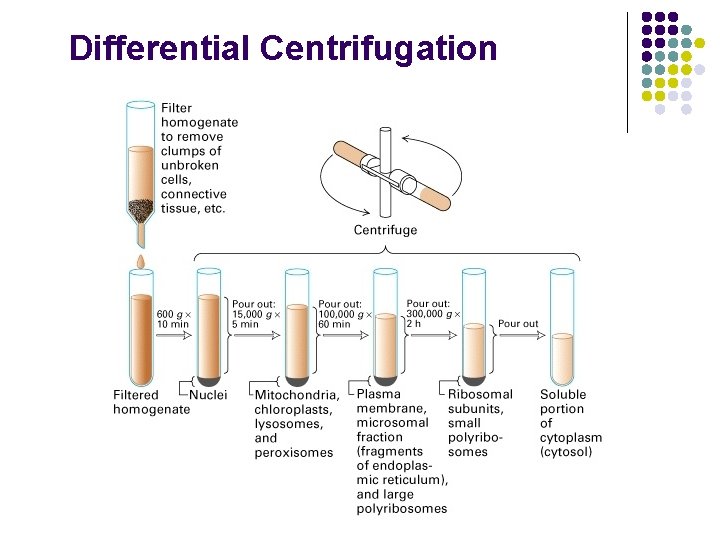
Differential Centrifugation
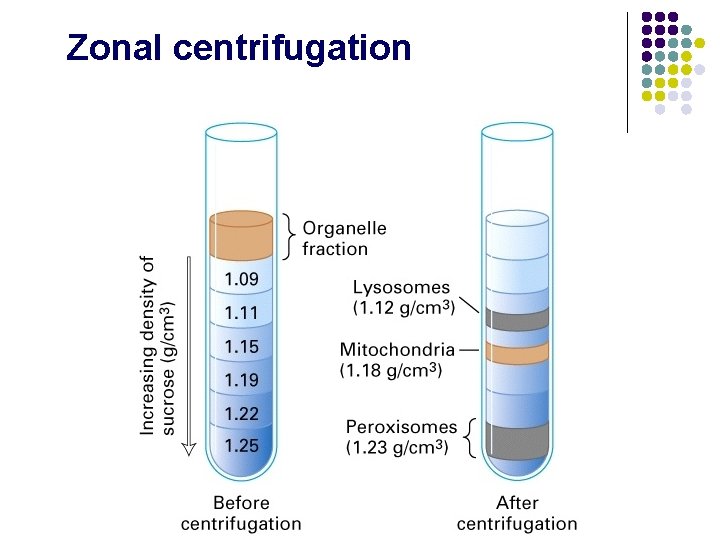
Zonal centrifugation
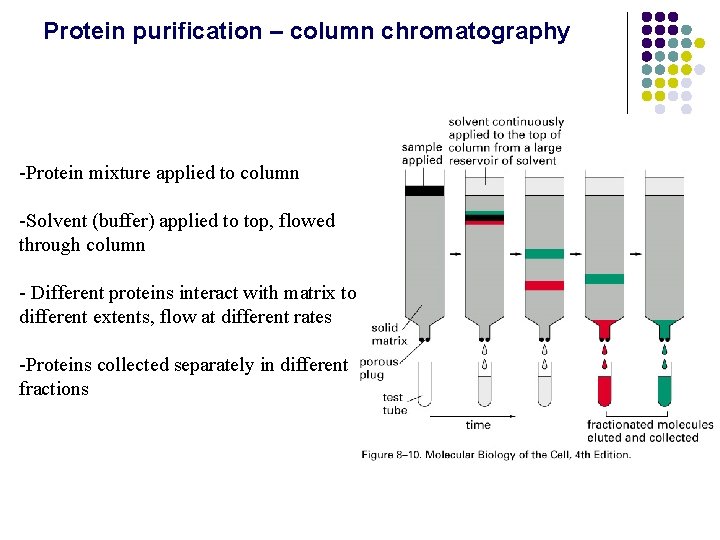
Protein purification – column chromatography -Protein mixture applied to column -Solvent (buffer) applied to top, flowed through column - Different proteins interact with matrix to different extents, flow at different rates -Proteins collected separately in different fractions
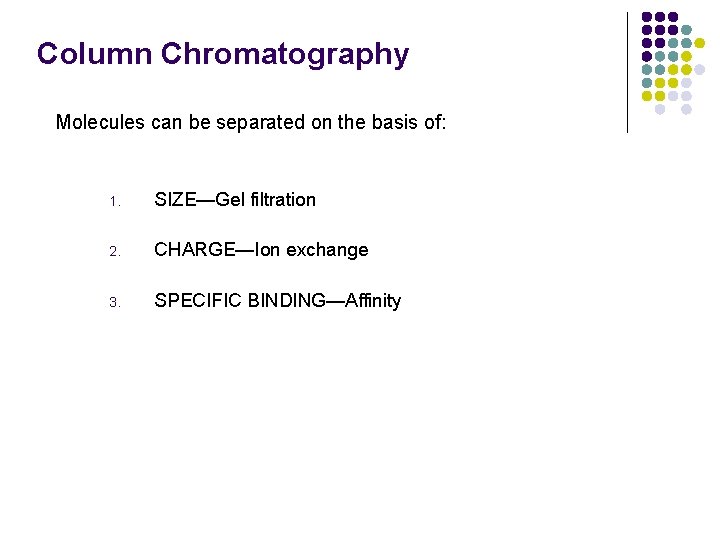
Column Chromatography Molecules can be separated on the basis of: 1. SIZE—Gel filtration 2. CHARGE—Ion exchange 3. SPECIFIC BINDING—Affinity
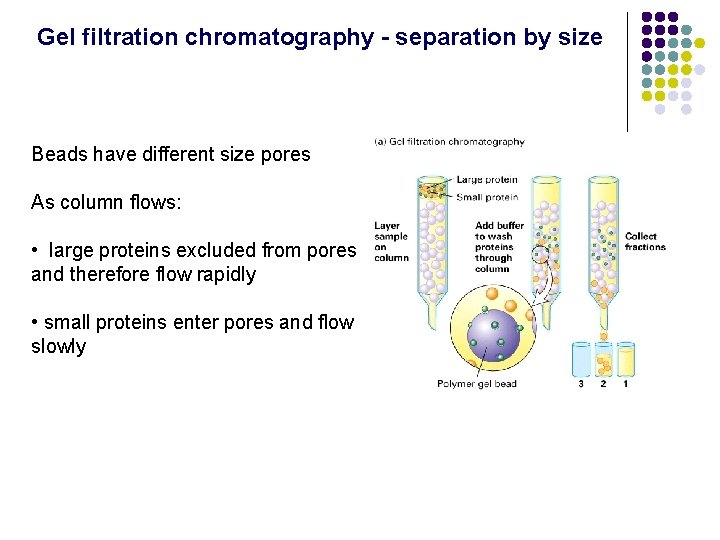
Gel filtration chromatography - separation by size Beads have different size pores As column flows: • large proteins excluded from pores and therefore flow rapidly • small proteins enter pores and flow slowly
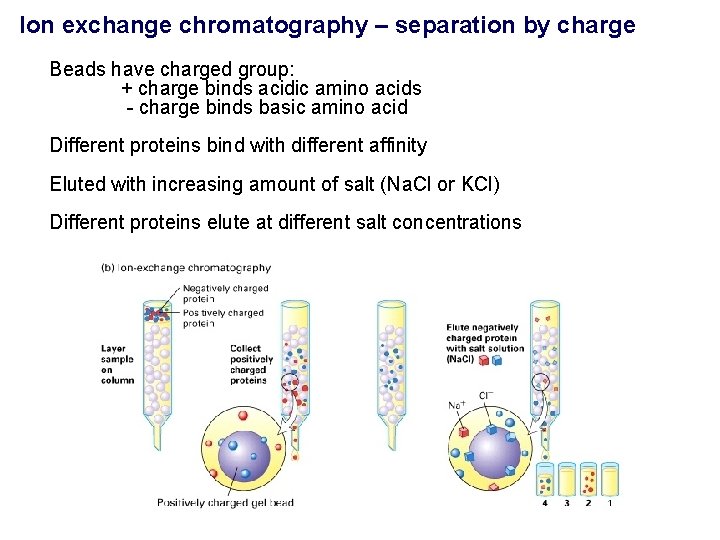
Ion exchange chromatography – separation by charge Beads have charged group: + charge binds acidic amino acids - charge binds basic amino acid Different proteins bind with different affinity Eluted with increasing amount of salt (Na. Cl or KCl) Different proteins elute at different salt concentrations
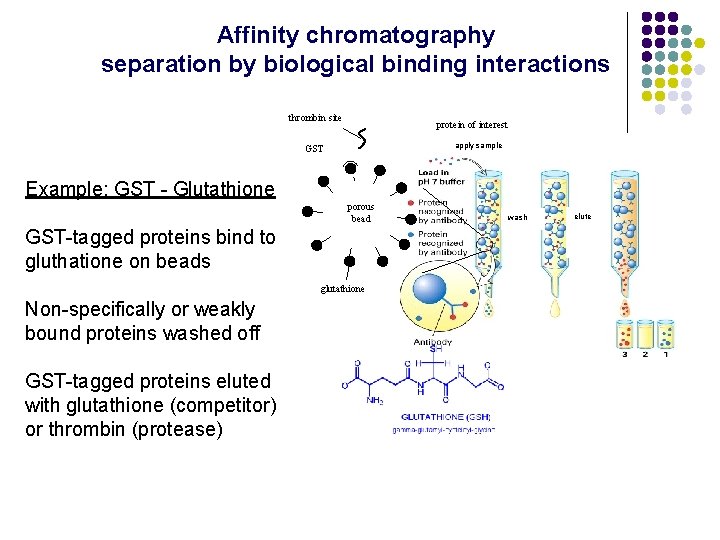
Affinity chromatography separation by biological binding interactions thrombin site protein of interest apply sample GST Example: GST - Glutathione porous bead GST-tagged proteins bind to gluthatione on beads glutathione Non-specifically or weakly bound proteins washed off GST-tagged proteins eluted with glutathione (competitor) or thrombin (protease) wash elute
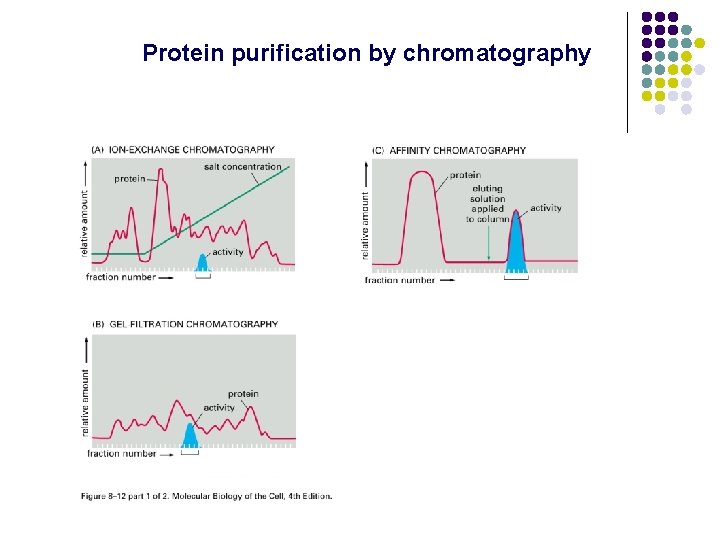
Protein purification by chromatography
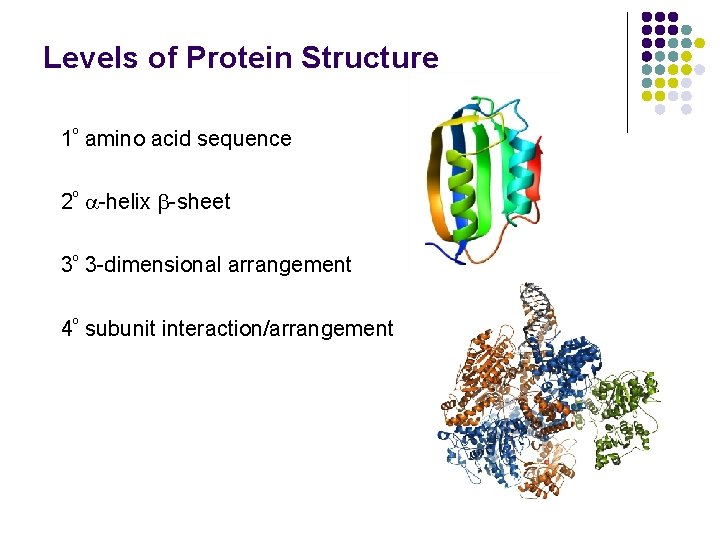
Levels of Protein Structure 1º amino acid sequence 2º -helix -sheet 3º 3 -dimensional arrangement 4º subunit interaction/arrangement
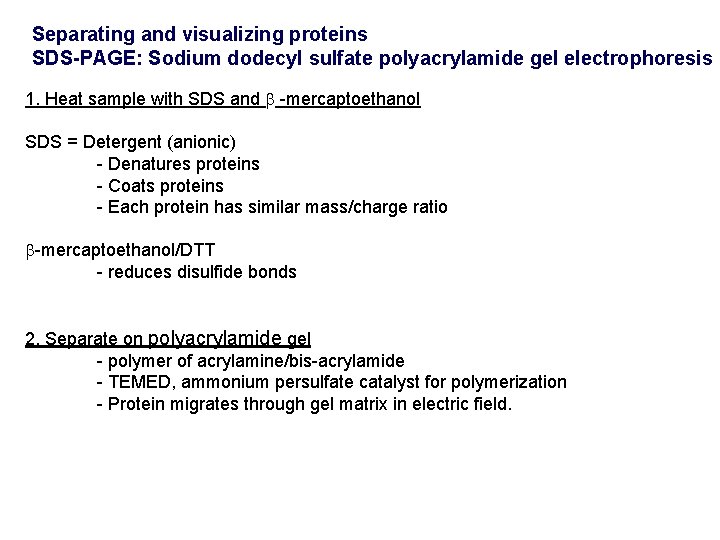
Separating and visualizing proteins SDS-PAGE: Sodium dodecyl sulfate polyacrylamide gel electrophoresis 1. Heat sample with SDS and -mercaptoethanol SDS = Detergent (anionic) - Denatures proteins - Coats proteins - Each protein has similar mass/charge ratio -mercaptoethanol/DTT - reduces disulfide bonds 2. Separate on polyacrylamide gel - polymer of acrylamine/bis-acrylamide - TEMED, ammonium persulfate catalyst for polymerization - Protein migrates through gel matrix in electric field.
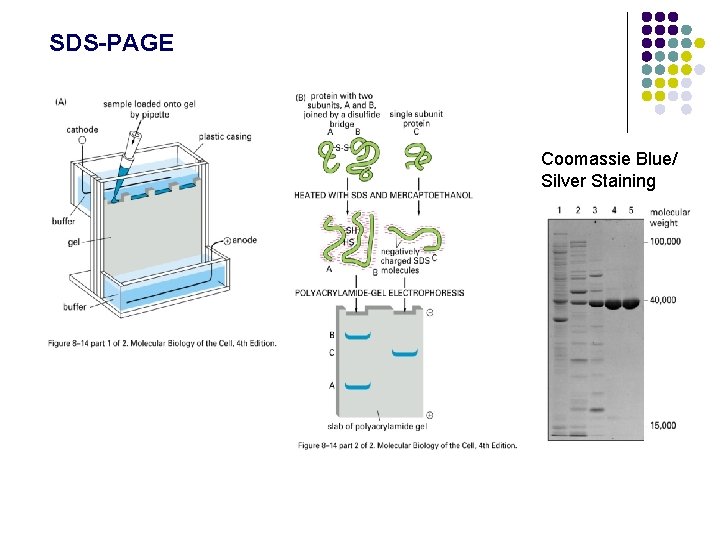
SDS-PAGE Coomassie Blue/ Silver Staining

SDS-PAGE movie http: //sdspage. homestead. com/The. Video. html


- Slides: 23