PROTEIN PENGERTIAN PROTEIN SECARA STRUKTUR Polymer of amino










- Slides: 36

PROTEIN

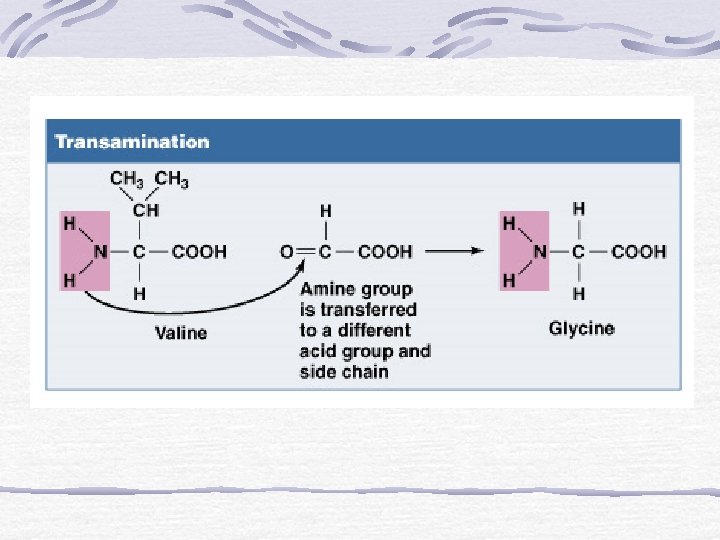
PENGERTIAN PROTEIN SECARA STRUKTUR: Polymer of amino acids amine group (N) acid group side chain

SECARA FUNGSI: Muscle fiber protein: Connective proteins others

Protein daging Secara kimiawi: tersusun atas: OTOT 1. myofibrillar: myosin 55%, (actin, troponin, tropomyosin 4045%), protein lain-2: 1 -5%, sifat kimia molekul protein ini: polar 2. Stromal (Connective tissue): jaringan ikat: TERSUSUN ATAS KOLAGEN: 10 -15%, protein ini berfungsi sebagai pengikat otot daging, menyalurkan tenaga dan mengikat otot daging shg tidak lepas dari kerangka tulang. Sifat kimia protein ini NON-POLAR: GLYCINE & hidroksi prolin 3. Sarcoplasmic protein (Water soluble protein): sebagai protein terlarut: berfungsi sebagai tempat aktifitas ensim protein dan warna (haemoglobin= warna merah daging)

Protein Structure Polymer of amino acids amine group (N) acid group side chain

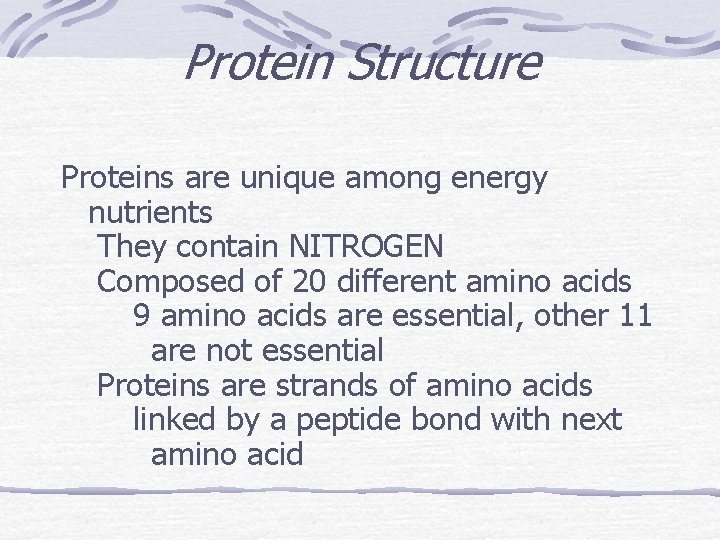
Protein Structure Proteins are unique among energy nutrients They contain NITROGEN Composed of 20 different amino acids 9 amino acids are essential, other 11 are not essential Proteins are strands of amino acids linked by a peptide bond with next amino acid


Glucose Triglyceride

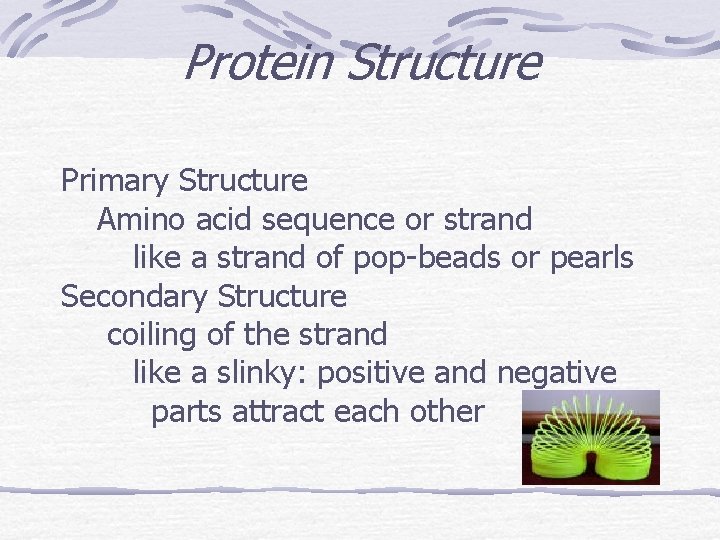
Protein Structure Primary Structure Amino acid sequence or strand like a strand of pop-beads or pearls Secondary Structure coiling of the strand like a slinky: positive and negative parts attract each other

Protein Structure Tertiary or third level of structure Folding back of coil The slinky gets messed up Quaternary or fourth level of structure Subunits fit together Hemoglobin has four subunits to make the functional molecule

Protein Structure SHAPE DETERMINES FUNCTION The shape of the protein molecule determines if the molecule is functional the shape of the lipase molecule determines if it will actually help breakdown a lipid

Protein Structure Change of shape is called DENATURATION What causes change of shape? acid (like the stomach low p. H) or base(high p. H) alcohol mechanical agitation(beating an egg white) heat(heat an egg white) or heavy metals(mercury)

KLASIFIKASI Berdasarkan bentuknya protein : 1. Protein bentuk serabut (fibrous) Protein ini terdiri atas beberapa rantai peptida berbentu spiral yang terjalin. Satu sama lain sehingga menyerupai batang yang kaku. Karakteristik protein bentuk serabut adalah rendahnya daya larut, mempunyai kekuatan mekanis yang tinggi untuk tahan terhadap enzim pencernaan. Kolagen merupakan protein utama jaringan ikat. Elasti terdapat dalam urat, otot, arteri (pembuluh darah) dan jaringan elastis lain. Keratini adalah protein rambut dan kuku. Miosin merupakan protein utama serat otot.

2. Protein globuler Berbentuk bola terdapat dalam cairan jaringan tubuh. Protein ini larut dalam larutan garam dan encer, mudah berubah dibawah pengaruh suhu, konsentrasi garam dan mudah denaturasi. Albumin terdapat dalam telur, susu, plasma, dan hemoglobin. Globulin terdapat dalam otot, serum, kuning telur, dan gizi tumbuh-tumbuhan. Histon terdapat dalam jaringan-jaringan seperti timus dan pancreas. Protamin dihubungkan dengan asam nukleat.

3. Protein konjugasi Merupakan protein sederhana yang terikat dengan baha-bahan non-asam amino. Nukleoprotein terdaoat dalam inti sel dan merupakan bagian penting DNA dan RNA. Nukleoprotein adalah kombinasi protein dengan karbohidrat dalam jumlah besar. Lipoprotein terdapat dalam plasma-plasma yang terikat melalui ikatan ester dengan asam fosfat sepertu kasein dalam susu. Metaloprotein adalah protein yang terikat dengan mineral seperti feritin dan hemosiderin adalah protein dimana mineralnya adalah zat besi, tembaga dan seng.

Denaturation

Goals • Denaturation • Balance of forces • Consequences of denaturation

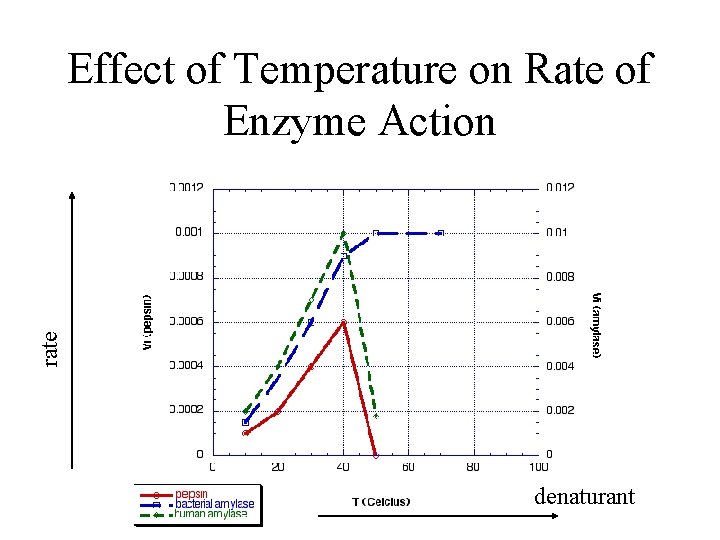
rate Effect of Temperature on Rate of Enzyme Action denaturant

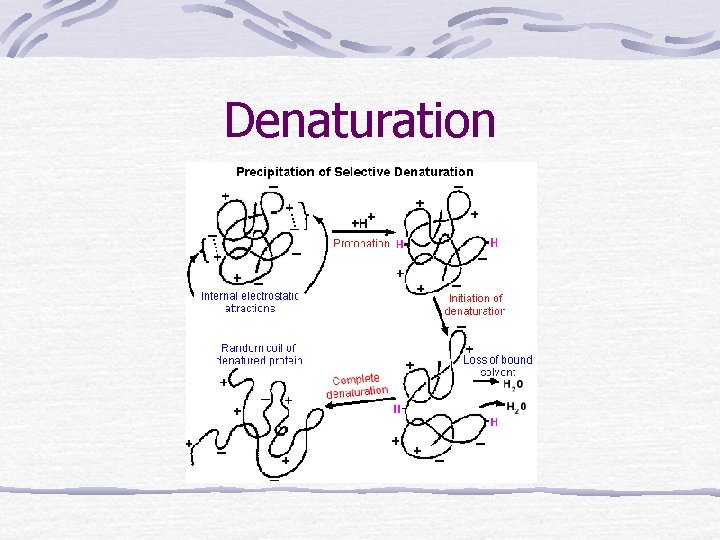
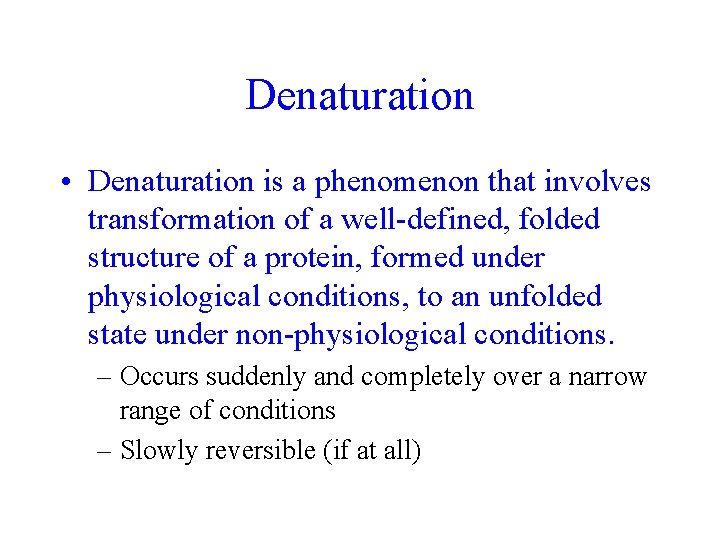
Denaturation • Denaturation is a phenomenon that involves transformation of a well-defined, folded structure of a protein, formed under physiological conditions, to an unfolded state under non-physiological conditions. – Occurs suddenly and completely over a narrow range of conditions – Slowly reversible (if at all)

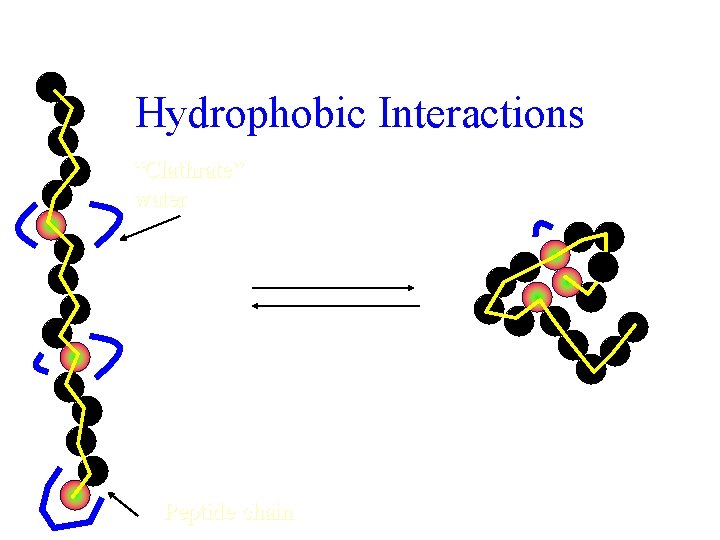
Hydrophobic Interactions “Clathrate” water Increased solvent entropy Increased chain entropy Peptide chain

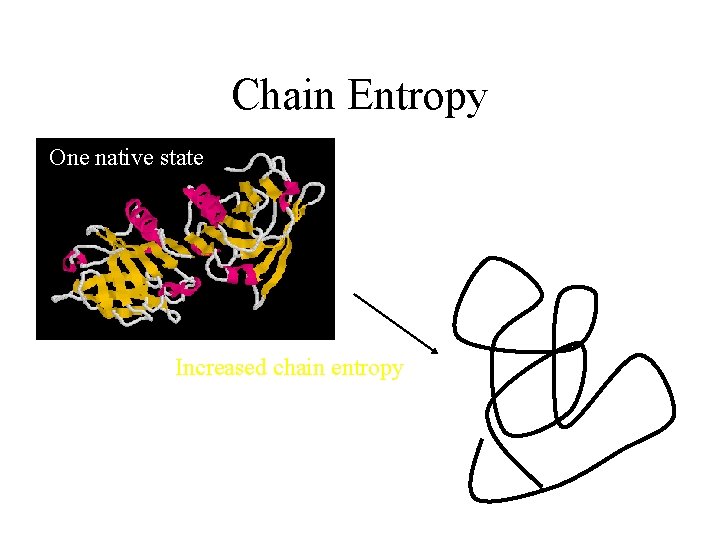
Chain Entropy One native state S=k ln W Increased chain entropy Many denatured states

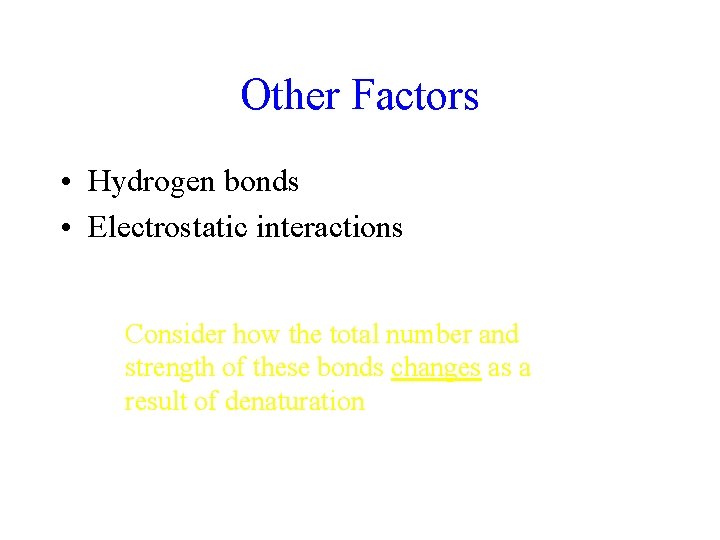
Other Factors • Hydrogen bonds • Electrostatic interactions Consider how the total number and strength of these bonds changes as a result of denaturation

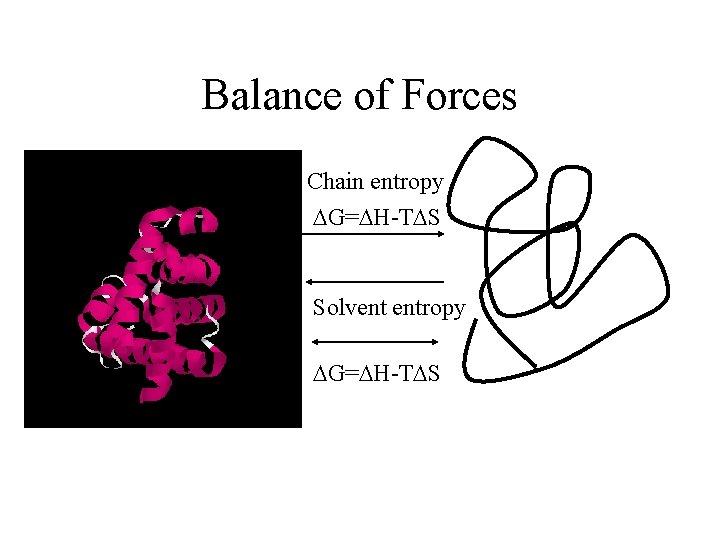
Balance of Forces Chain entropy DG=DH-TDS Solvent entropy DG=DH-TDS other forces

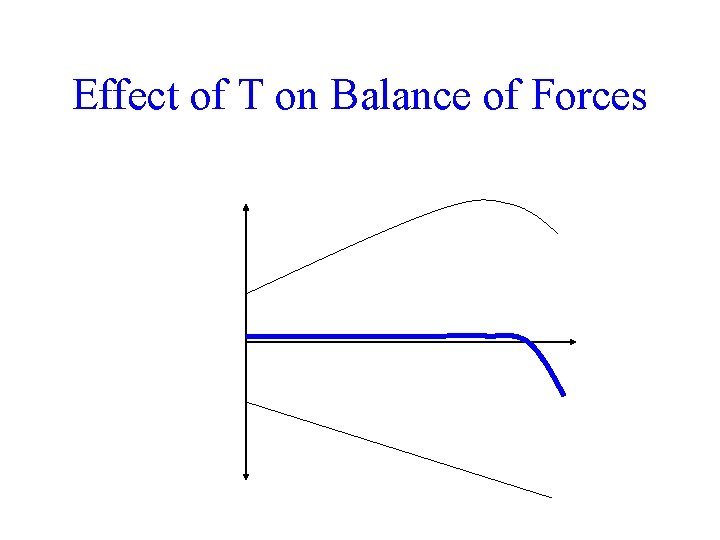
+ (oppose) - (favor) Free energy change for denaturation Effect of T on Balance of Forces Solvent entropy effect T Chain entropy effect

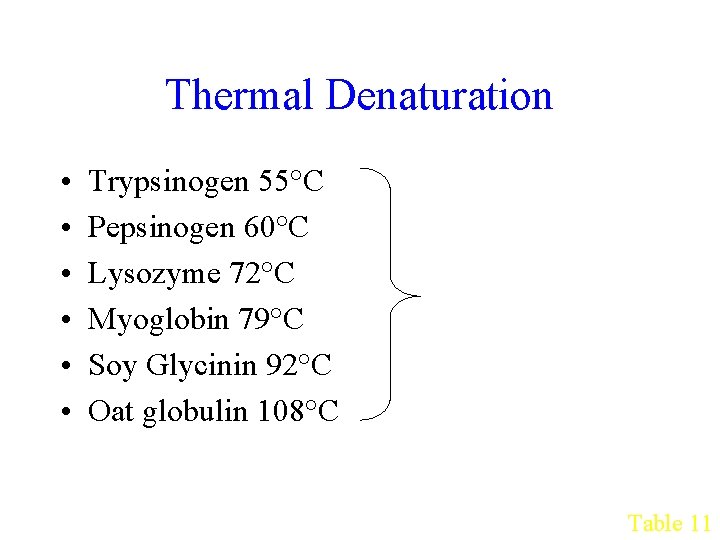
Thermal Denaturation • • • Trypsinogen 55°C Pepsinogen 60°C Lysozyme 72°C Myoglobin 79°C Soy Glycinin 92°C Oat globulin 108°C Affected by p. H, water, solutes Table 11

Types of Denaturation • • • Temperature Organic solvents Surface p. H Shear

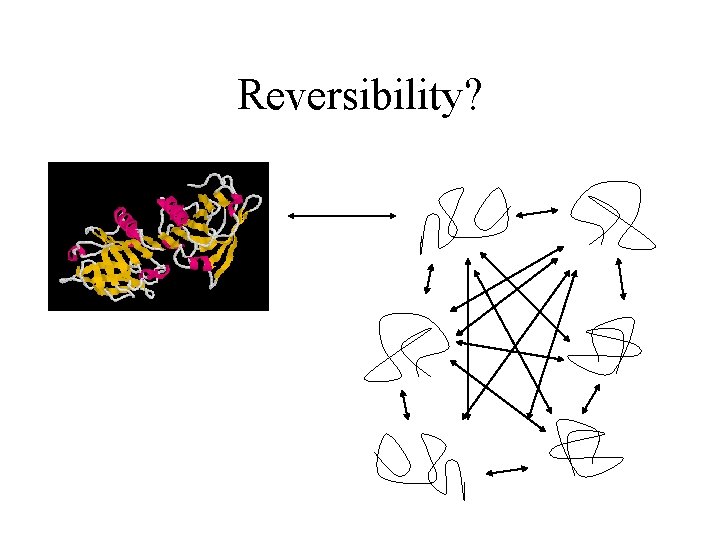
Reversibility? One native form Refolding is a complex process – particularly for large proteins or complex proteins Many denatured forms

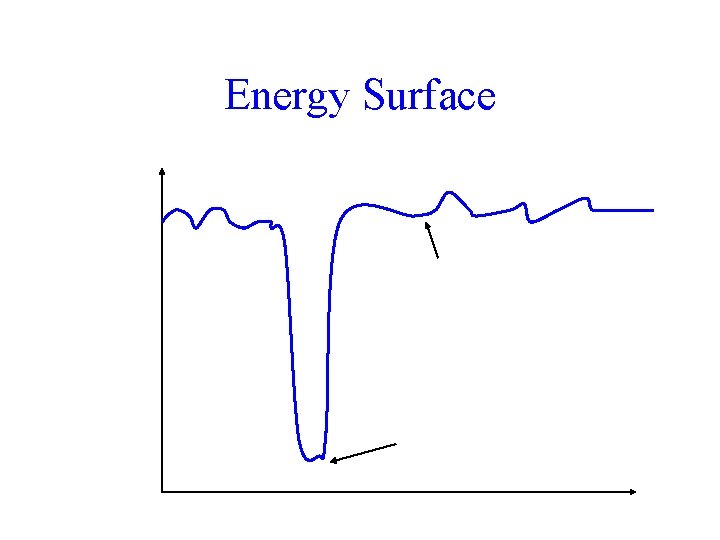
Free energy Energy Surface Many secondary minima amongst denatured states One native state (true energy minimum) Changes in Conformation

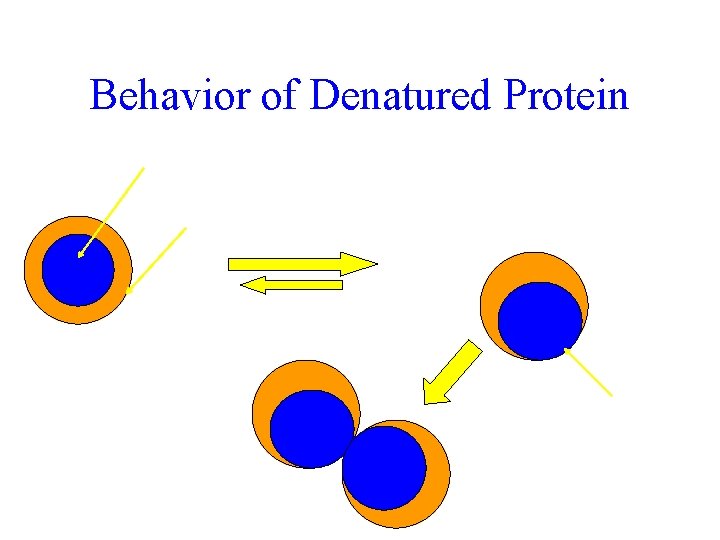
Behavior of Denatured Protein Hydrophobic core Hydrophilic surface Fast under non-physiological conditions DENATURED Slow under physiological conditions NATIVE AGGREGATED or other ingredient interactions Unfolding forces some hydrophobic AA to surface

Consequences of Denaturation • • • Loss of enzymatic activity (death) Destruction of toxins Improved digestibility Loss of solubility Changes in texture

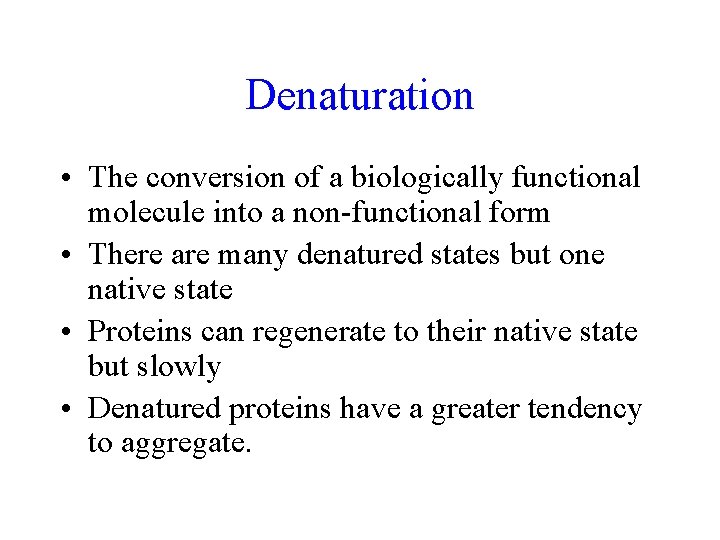
Denaturation • The conversion of a biologically functional molecule into a non-functional form • There are many denatured states but one native state • Proteins can regenerate to their native state but slowly • Denatured proteins have a greater tendency to aggregate.

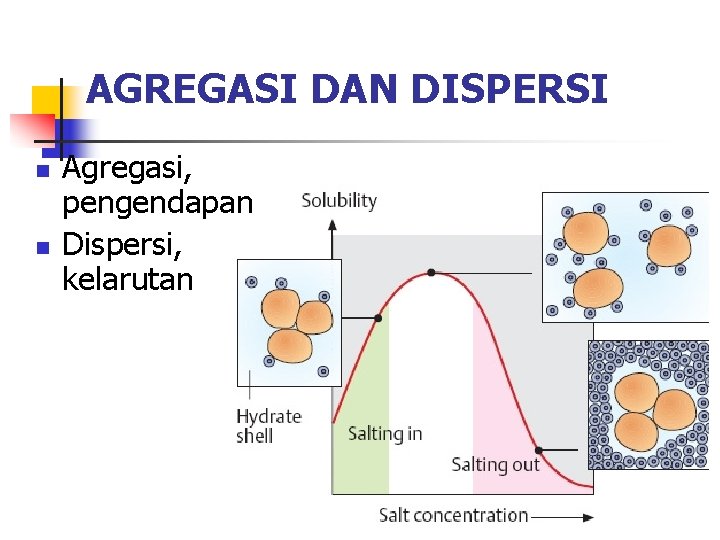
AGREGASI DAN DISPERSI n n Agregasi, pengendapan Dispersi, kelarutan

KELARUTAN PROTEIN Salting In Penambahan garam dengan kekuatan ion yang rendah dapat meningkatkan kelarutan protein dengan menetralkan muatan di permukaan protein, sehingga mengurangi air yang menyelimuti protein dan meningkatkan entropi sistem.

PENGENDAPAN PROTEIN Salting out (digunakan pada Fraksinasi) Jika konsentrasi garam netral berada pada tingkatan yang tinggi (>0, 1 M), dalam banyak kasus protein menjadi mengendap. Hal ini disebabkan keberadaan ion (yang tidak terikat protein) dalam jumlah yang banyak berkompetisi dengan protein untuk mendapatklan pelarut. Penurunan jumlah pelarut yang tersedia menyebabkan protein beragregasi dan mengendap.

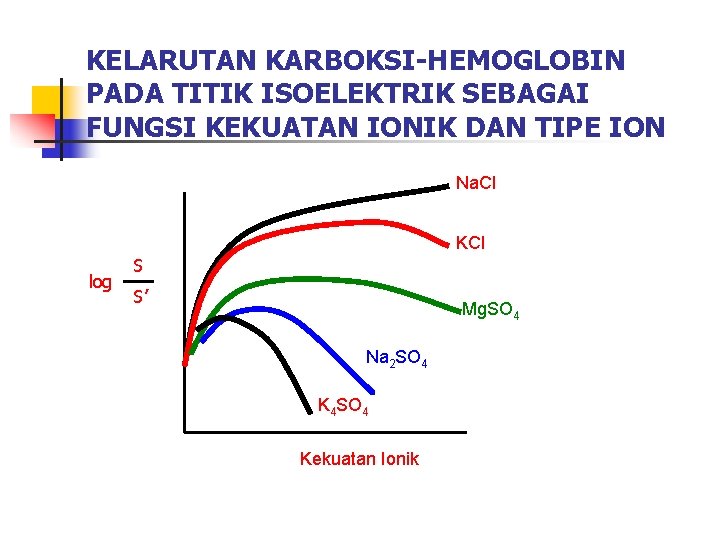
KELARUTAN KARBOKSI-HEMOGLOBIN PADA TITIK ISOELEKTRIK SEBAGAI FUNGSI KEKUATAN IONIK DAN TIPE ION Na. Cl KCl S log S’ Mg. SO 4 Na 2 SO 4 K 4 SO 4 Kekuatan Ionik

STABILISASI PROTEIN Protein paling stabil jika berada pada lingkungan dengan p. H da kekuatan ionik yang mendekati kondisi fisiologis. p. H: 7, 4, (enzim lisosom, p. H 5) I (kekuatan ionik) = 0, 15 M