PROTEIN MODELING CHALLENGE Study the structure and function











- Slides: 49

PROTEIN MODELING CHALLENGE Study the structure and function of the protein: ABO Blood typing glycosyltransferases Utilize primary peer-reviewed literature and molecule of the month article Use the protein Data Base (PDB. org) to obtain structural information Construct a 3 -D model using a foam toober and the imaging program Jmol. Write an essay about the protein structure, function and the model your group makes.


PLAN OF ACTION PROTEIN BIOCHEMISTRY 11/17 -11/25 (4 days) ◦ Protein Structure ◦ Protein Side-Chains ◦ Protein Biochemistry 12/1 – 12/10 (6 days) ◦ Jmol tutorial ◦ Practice using Jmol ◦ Model simulations Beta-globulin Zinc fingers Op. Ca protein

PROTEIN MODELING TEAM 12/11 – 12/19 (~5 class days) 1/5 – 1/16 (~10 class days) 1/19– 1/30 (~10 class days) ◦ Research Articles on the protein ◦ Write a draft abstract about the protein – DUE 12/18 & 12/19 ◦ Develop a model of the protein in Jmol that highlights important parts ◦ Create a 3 -D model using foam toobers ◦ Construct Display for model ◦ Write final abstract – DUE 1/30/15 MODELS DUE 2/13/15 Protein Modeling Challenge ◦ Thursday 2/26/15 at SUNY Stony Brook

PROTEIN BIOCHEMISTRY 2014/15 Proteins and Organic Molecules

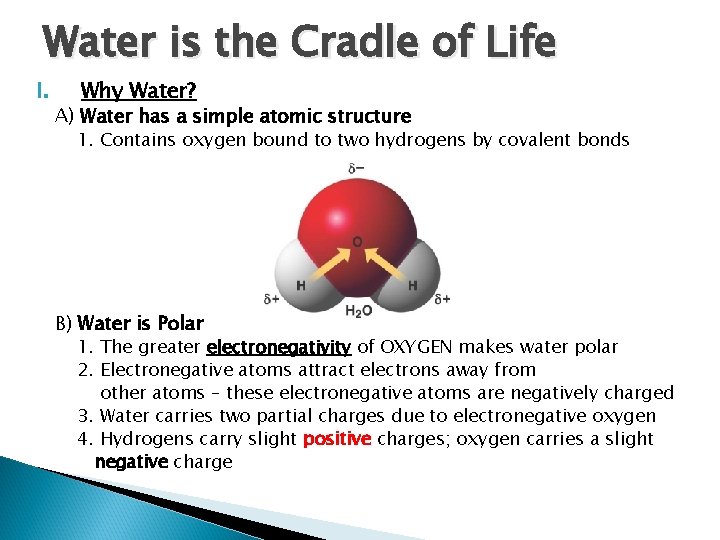
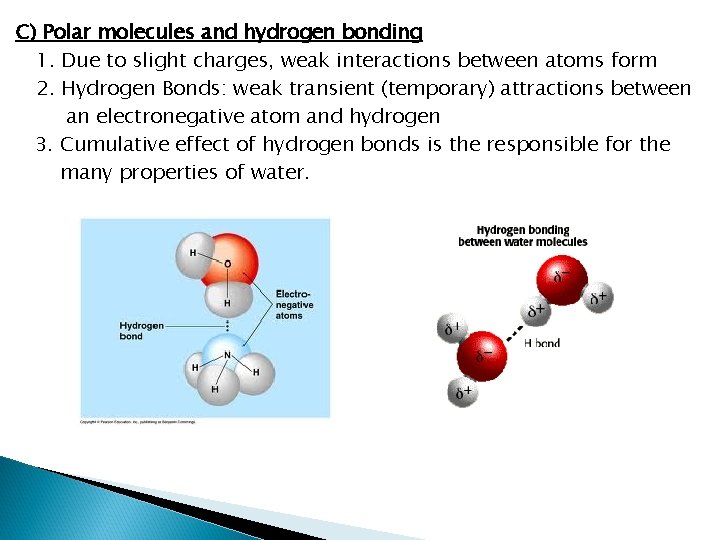
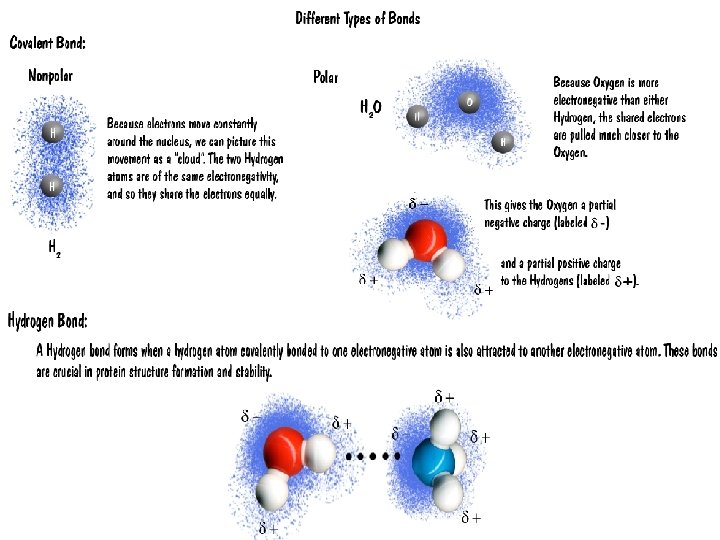
Water is the Cradle of Life I. Why Water? A) Water has a simple atomic structure 1. Contains oxygen bound to two hydrogens by covalent bonds B) Water is Polar 1. The greater electronegativity of OXYGEN makes water polar 2. Electronegative atoms attract electrons away from other atoms – these electronegative atoms are negatively charged 3. Water carries two partial charges due to electronegative oxygen 4. Hydrogens carry slight positive charges; oxygen carries a slight negative charge

C) Polar molecules and hydrogen bonding 1. Due to slight charges, weak interactions between atoms form 2. Hydrogen Bonds: weak transient (temporary) attractions between an electronegative atom and hydrogen 3. Cumulative effect of hydrogen bonds is the responsible for the many properties of water.

Why is water important to proteins? Proteins are made in cells which are comprised of 70% water. Polar nature of water plays a critical role in determining the final folded shape of a protein. Hydrogen bonding interactions directly determine the final structure of a protein Proteins fold in their lowest energy state – requires minimal bonding

Water Lover? Water Fearer? Hydrophobic ◦ Any molecule or atom that repels water ◦ Usually non-polar or uncharged Hydrophilic ◦ Any molecule or atom that attracts water ◦ Usually polar, charged, acid or base Hydrophobicity and hydrophilicity contribute to the overall protein structure

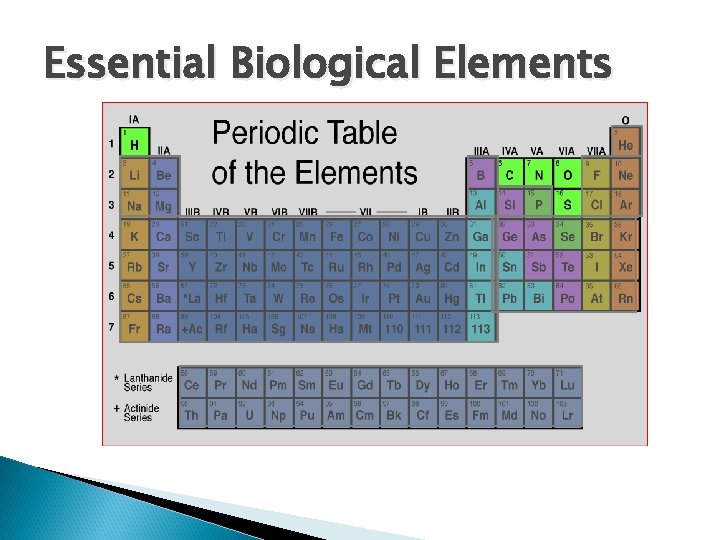
Essential Biological Elements

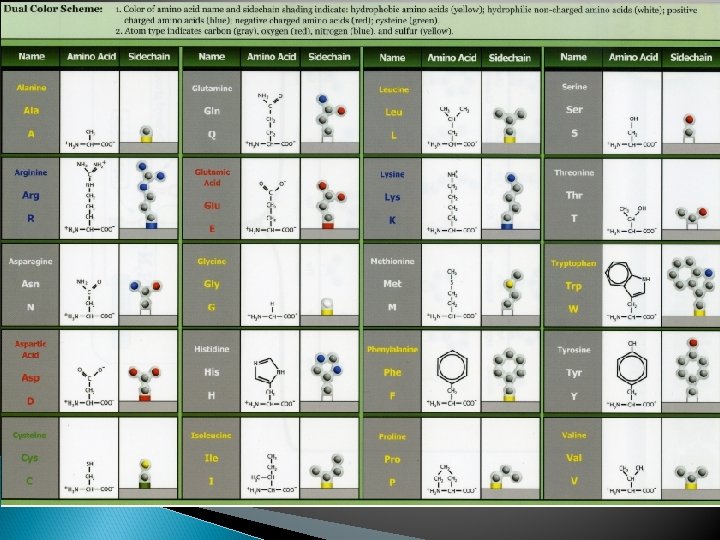
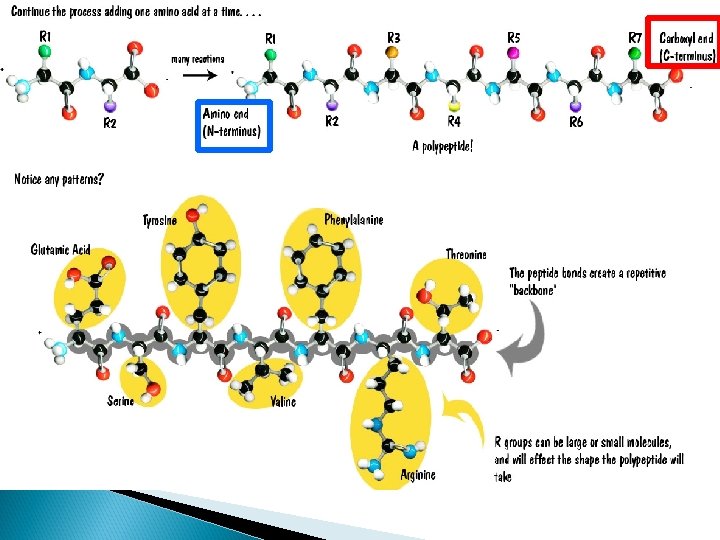
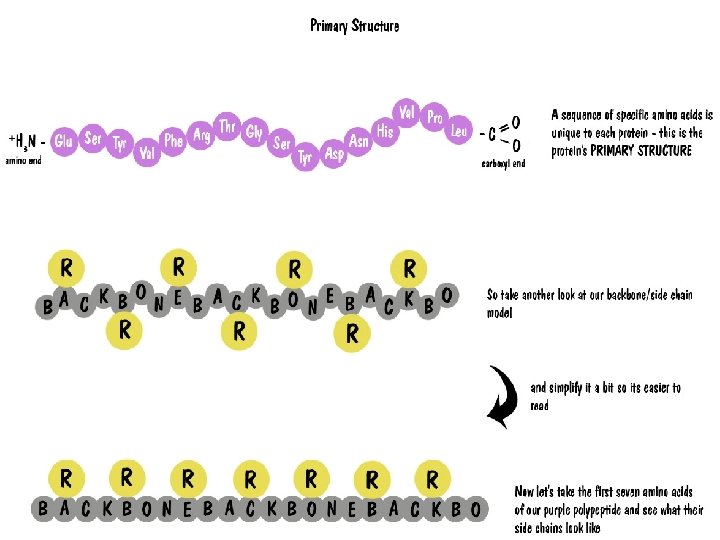
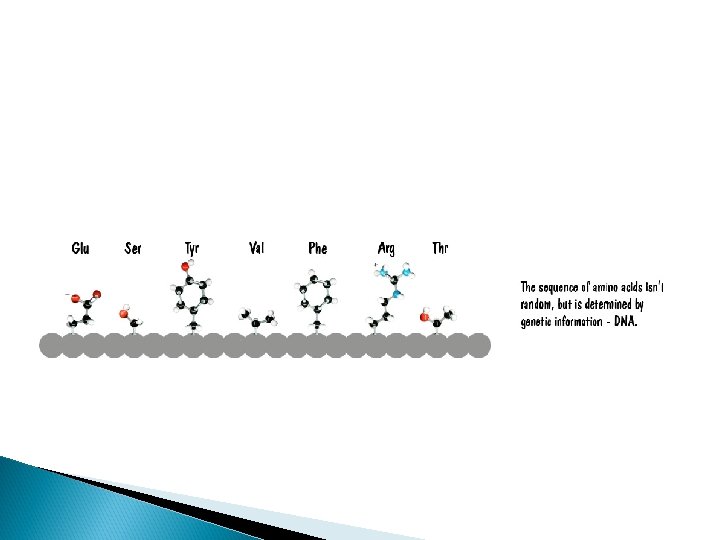
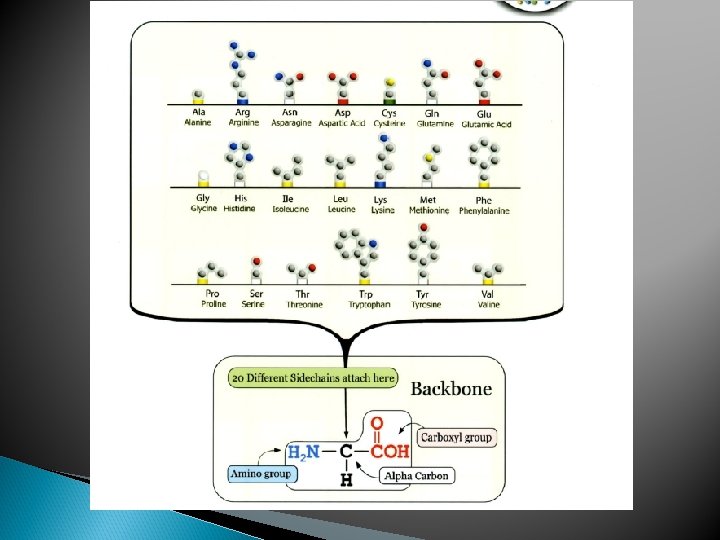
Proteins Polymers comprised of amino acid sequences The specific order of amino acids in a polypeptide interacts with the environment to determine the overall shape of the protein Amino acids contain three major reactive components The R group of an amino acid can be categorized by chemical properties Carboxyl group Amino Group Radical (R) group hydrophobic, hydrophilic, acidic, basic, or ionic R group interactions determine the structure and function of that region of the protein.

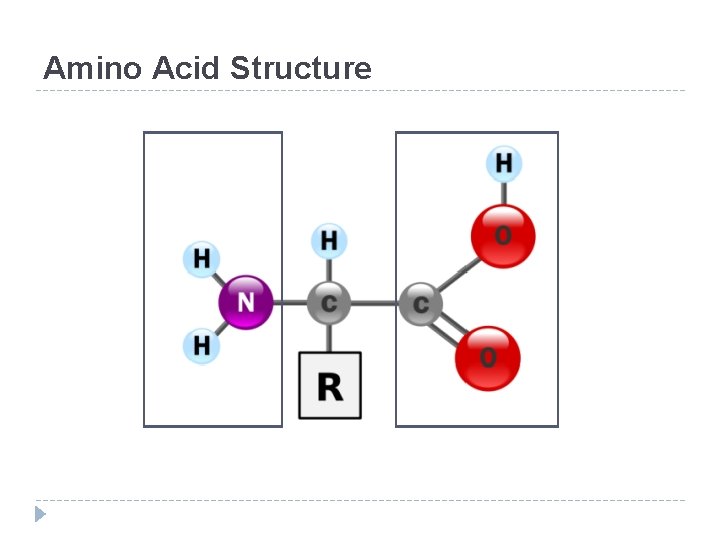
Amino Acid Structure


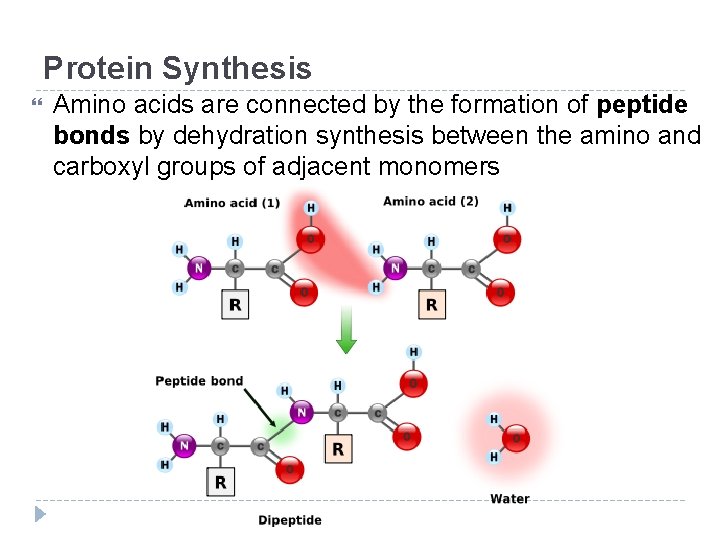
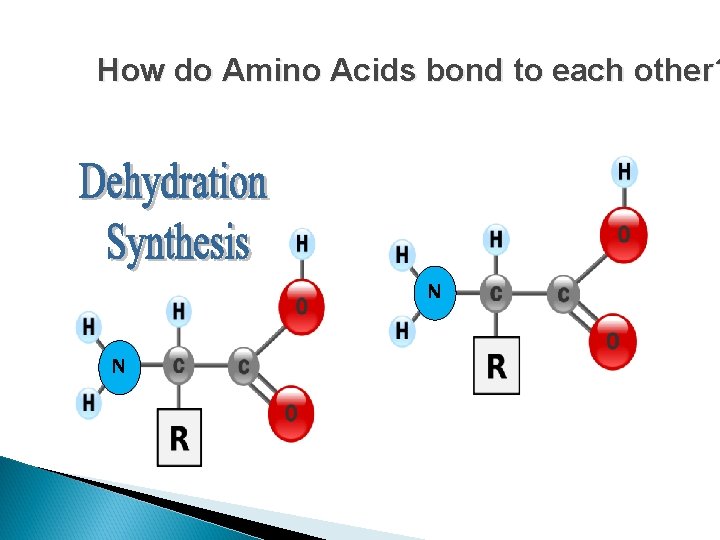
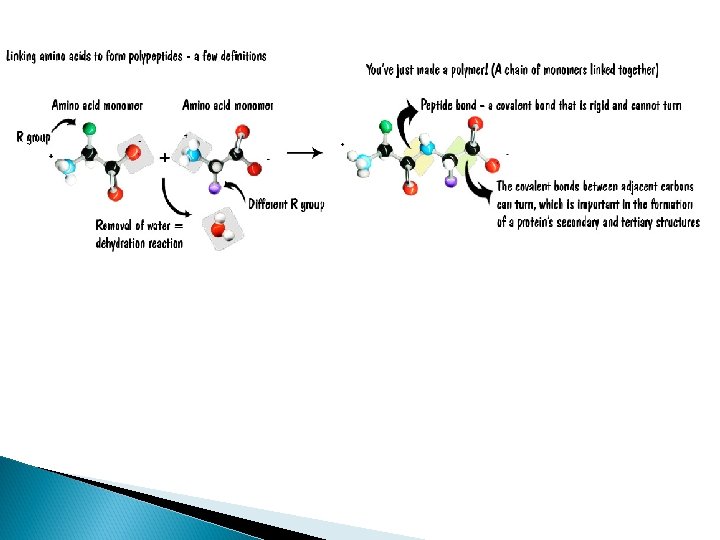
Protein Synthesis Amino acids are connected by the formation of peptide bonds by dehydration synthesis between the amino and carboxyl groups of adjacent monomers

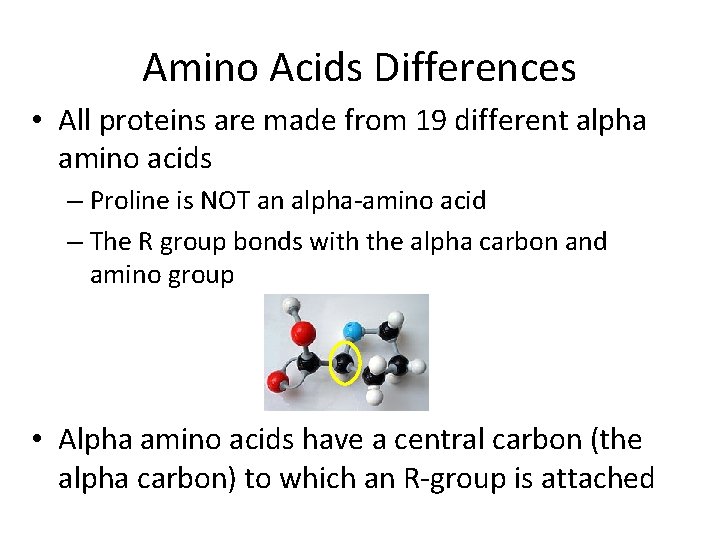
Amino Acids Differences • All proteins are made from 19 different alpha amino acids – Proline is NOT an alpha-amino acid – The R group bonds with the alpha carbon and amino group • Alpha amino acids have a central carbon (the alpha carbon) to which an R-group is attached

Moly Mod Kits Side chain Build 2 amino acids in your group


What group is circled?

What group is circled?

What is circled?

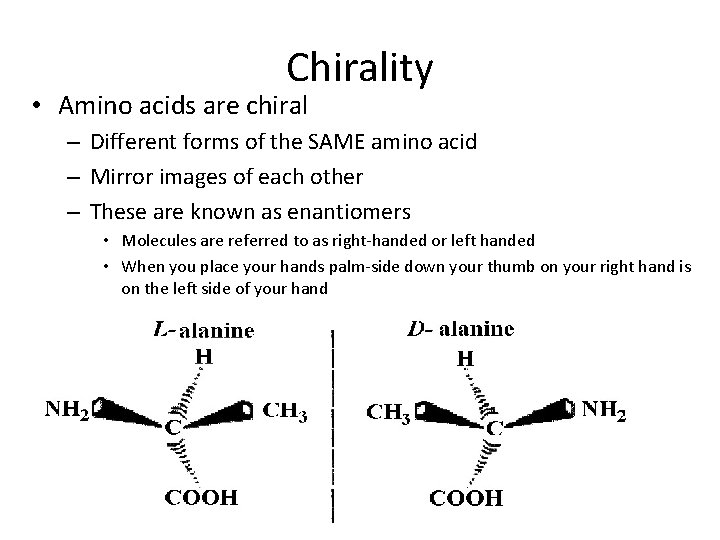
Chirality • Amino acids are chiral – Different forms of the SAME amino acid – Mirror images of each other – These are known as enantiomers • Molecules are referred to as right-handed or left handed • When you place your hands palm-side down your thumb on your right hand is on the left side of your hand

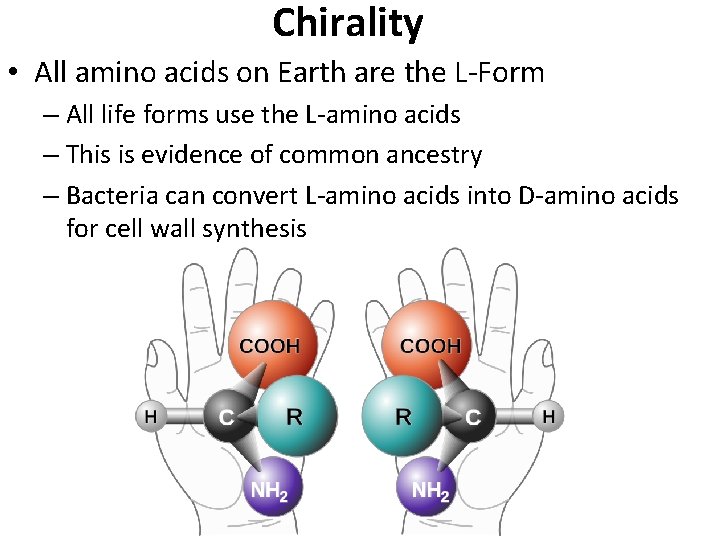
Chirality • All amino acids on Earth are the L-Form – All life forms use the L-amino acids – This is evidence of common ancestry – Bacteria can convert L-amino acids into D-amino acids for cell wall synthesis

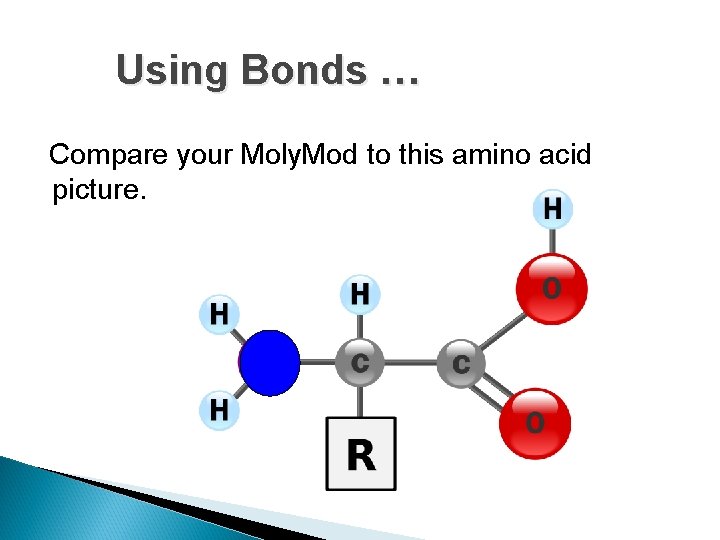
Using Bonds … Compare your Moly. Mod to this amino acid picture.

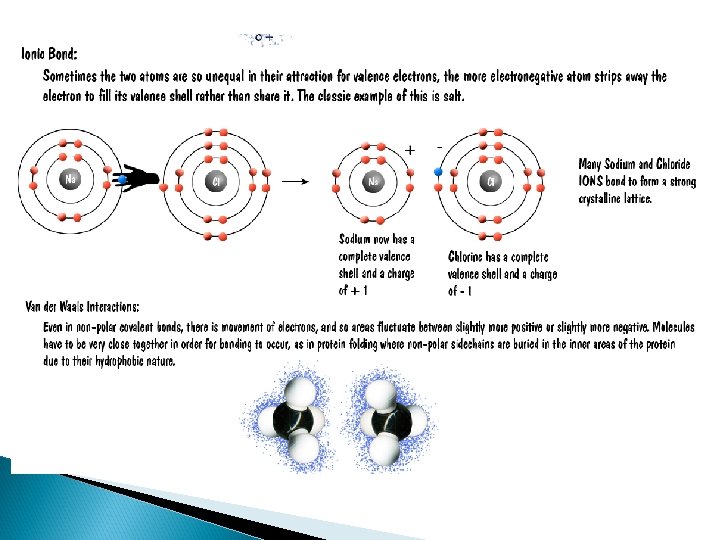
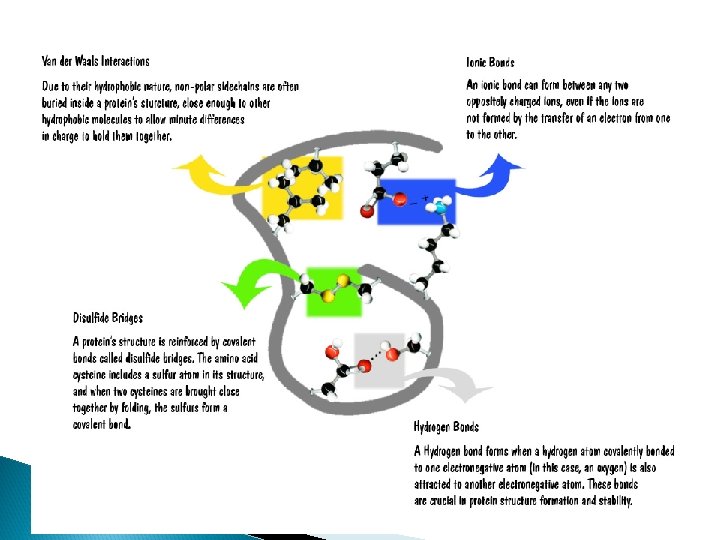
How are these things put together? ¡ BONDS! ¡ Are “highways” between 2 atoms. Bonds allow electron movement. ¡ We are concerned with THREE types of bonds: ¡ l l l Hydrogen Ionic Covalent

Covalent Bonds ¡ Two atoms share electrons l l All sharing is not equal. l When electrons are not shared equally it is called a polar covalent bond. When sharing is equal l When electrons are shared equally it is called a nonpolar covalent bond.

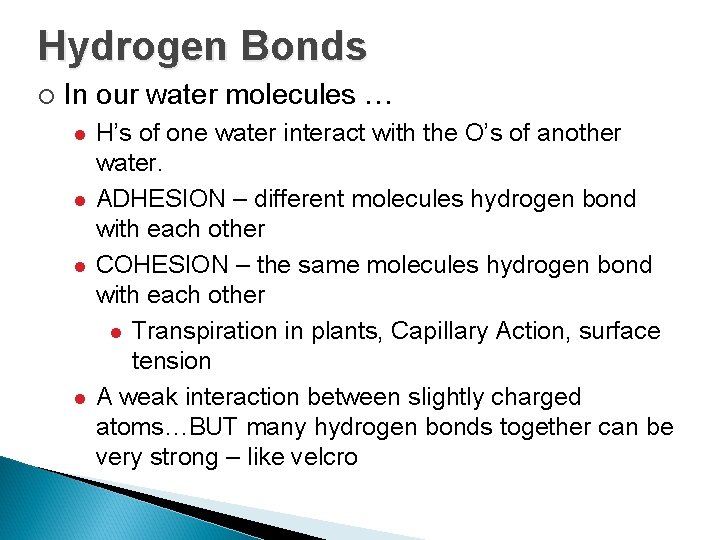
Hydrogen Bonds ¡ In our water molecules … l l H’s of one water interact with the O’s of another water. ADHESION – different molecules hydrogen bond with each other COHESION – the same molecules hydrogen bond with each other l Transpiration in plants, Capillary Action, surface tension A weak interaction between slightly charged atoms…BUT many hydrogen bonds together can be very strong – like velcro

Ionic ¡ One atom gives another atom their electrons ¡ Also called a salt bridge



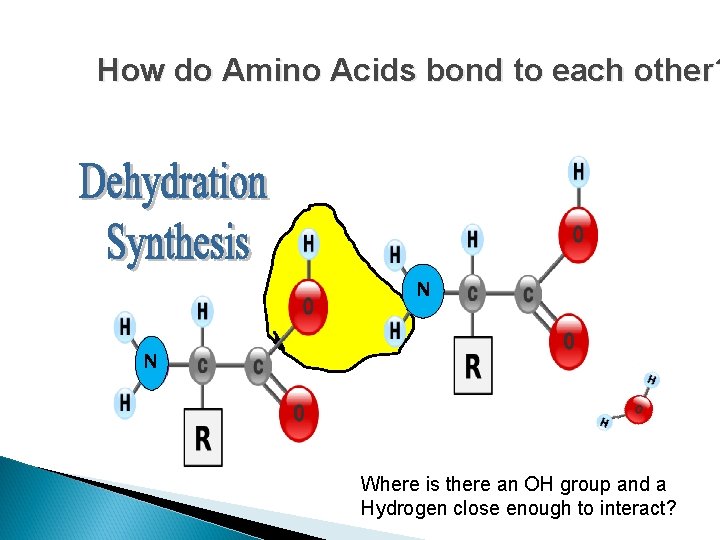
How do Amino Acids bond to each other? N N

How do Amino Acids bond to each other? The removal of a Hydrogen from one molecule and an “OH” from another molecule. This results in water as a product and …. A BOND BETWEEN THE TWO MOLECULES.

How do Amino Acids bond to each other? N N Where is there an OH group and a Hydrogen close enough to interact?











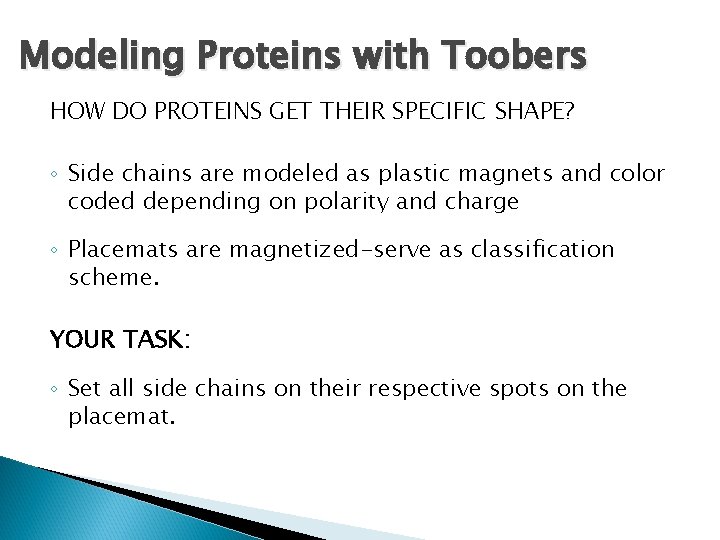
Modeling Proteins with Toobers HOW DO PROTEINS GET THEIR SPECIFIC SHAPE? ◦ Side chains are modeled as plastic magnets and color coded depending on polarity and charge ◦ Placemats are magnetized-serve as classification scheme. YOUR TASK: ◦ Set all side chains on their respective spots on the placemat.

Three basic principles of chemistry ◦ Hydrophobic amino acid side chains will cluster in the middle-less water interaction. Hydrophilic side chains including charged and polar will be exposed on the outside. ◦ When possible, opposite charges attract to form salt bridges. ◦ Cysteine side chains pair up to form disulfide bonds. . . are they hydrophobic or hydrophilic? ? ? ◦ Use the color chart and the magnetic placemat to help you determine side chain interactions

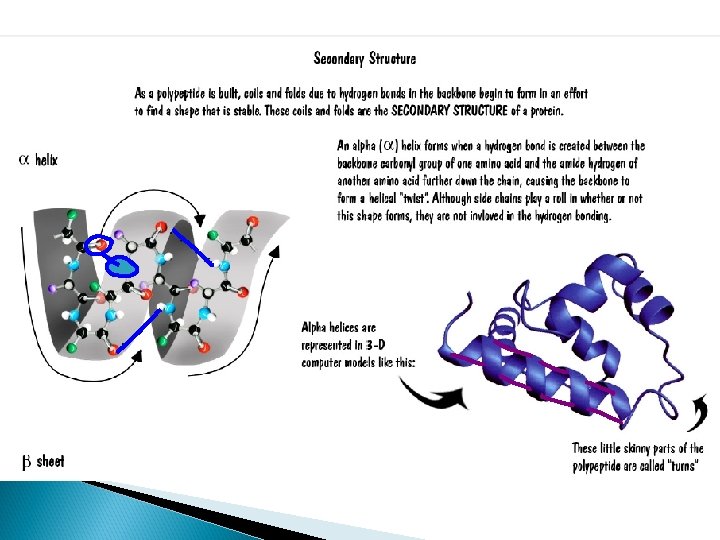
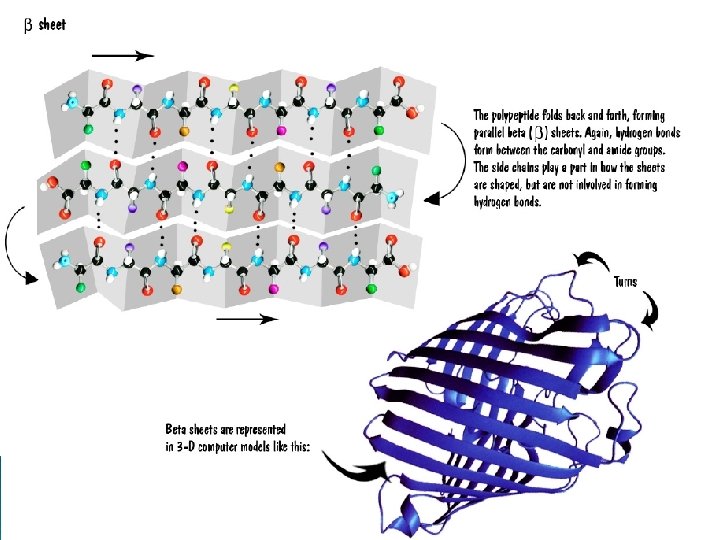
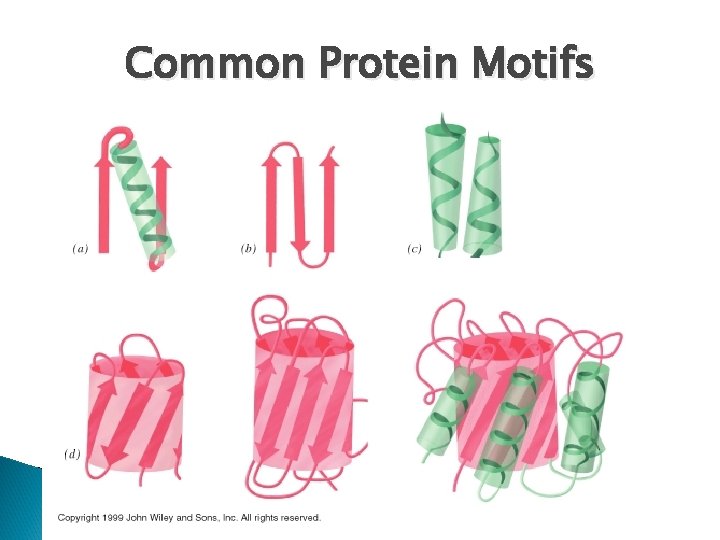
Important Terms to Know • General Protein Structure – Domain: A protein domain is a part of protein sequence and structure that can evolve, function, and exist independently of the rest of the protein chain – Motif: specific pattern of folding in a protein that is seen in several types of different proteins; motifs do not indicate similar function – these are common patterns – Lobe: area of a protein with a specific chemical nature • Hydrophobic lobe – Cleft: a specific area of a protein that forms a space between areas that may function as a regulatory site – Face: surface feature of a protein, usually formed by several beta-sheets

Back to the drawing board. Things to remember! ◦ The shape of the protein is pre-determined by the amino acid sequence. ◦ NOT ALL amino acid sequences are functional due to shape issues. A sequence may result in a terribly shaped protein ◦ Stability is KEY issue in protein folding!

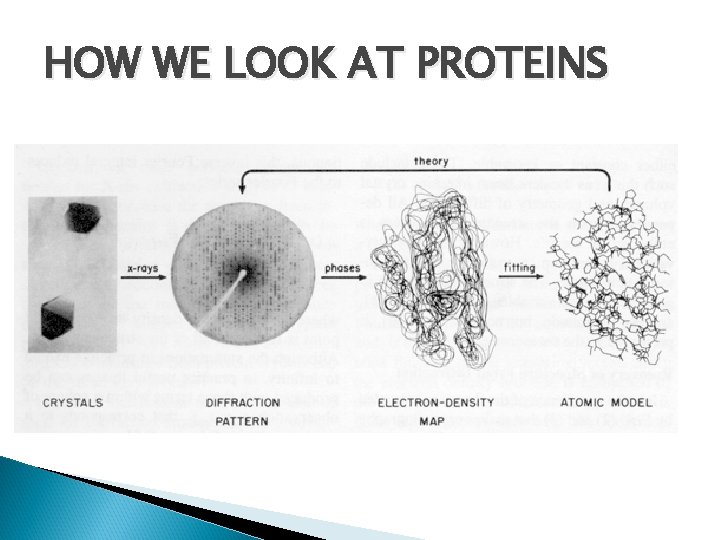
HOW WE LOOK AT PROTEINS

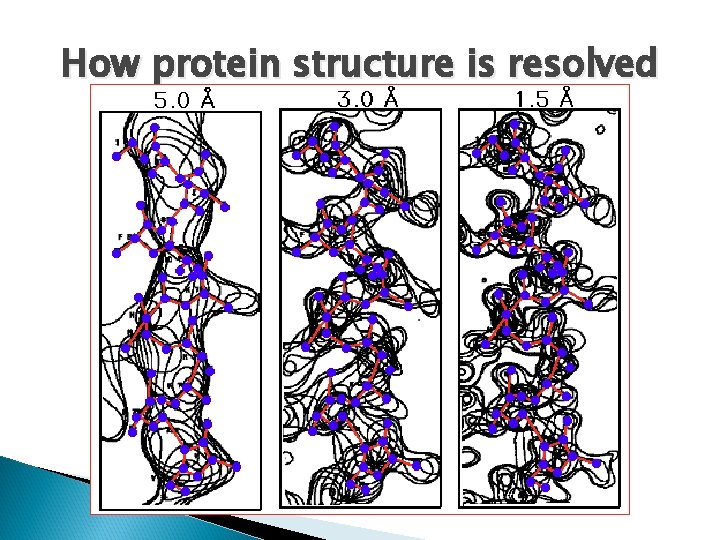
How protein structure is resolved

Common Protein Motifs