Protein Engineering n Protein engineering Generation of proteins


- Slides: 16

Protein Engineering n Protein engineering ¨ Generation of proteins suitable for industrial applications ¨ Thermostability, resistance to organic solvent etc. n Industrial enzymes (Table 8. 1)

Protein Engineering n n n n n Adding disulfide bonds Changing Asn to other amino acids Reducing the number of free sulfhydryl residues Increasing enzyme activity Modifying metal cofactor requirements Decreasing protease sensitivity Modifying protein specificity Increasing enzyme stability and specificity Altering multiple properties

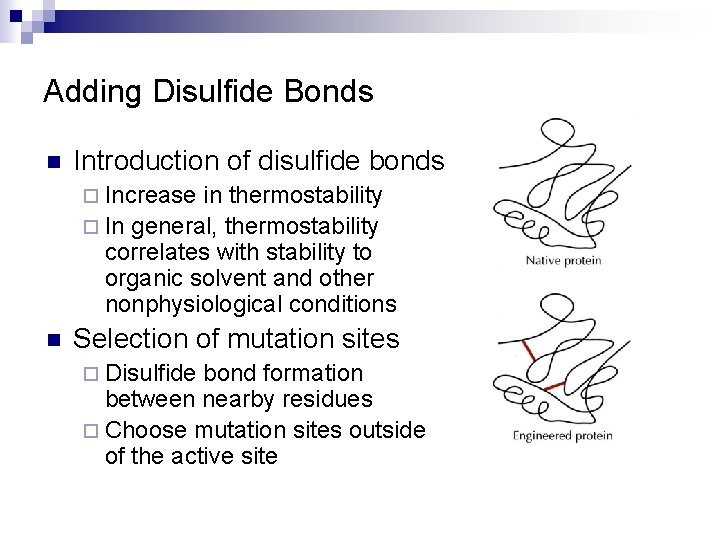
Adding Disulfide Bonds n Introduction of disulfide bonds ¨ Increase in thermostability ¨ In general, thermostability correlates with stability to organic solvent and other nonphysiological conditions n Selection of mutation sites ¨ Disulfide bond formation between nearby residues ¨ Choose mutation sites outside of the active site

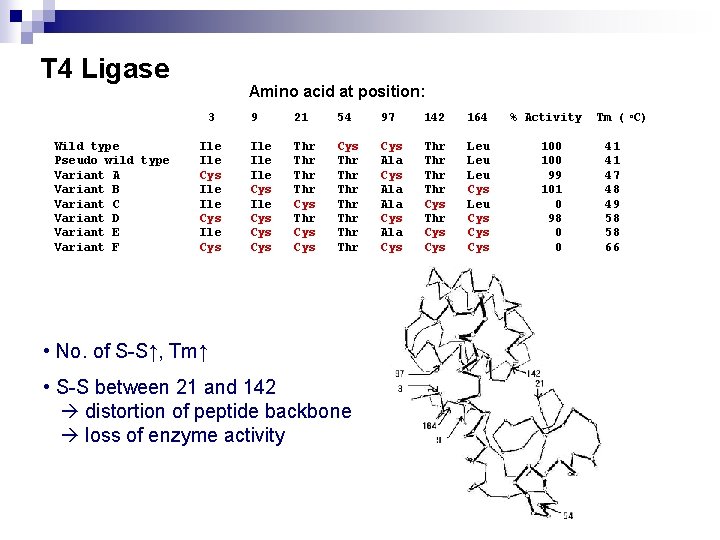
T 4 Ligase Amino acid at position: 3 Wild type Pseudo wild type Variant A Variant B Variant C Variant D Variant E Variant F Ile Ile Cys 9 21 54 97 142 164 Ile Ile Cys Cys Thr Thr Cys Cys Thr Thr Cys Ala Ala Cys Thr Thr Cys Leu Leu Cys Cys • No. of S-S↑, Tm↑ • S-S between 21 and 142 distortion of peptide backbone loss of enzyme activity % Activity 100 99 101 0 98 0 0 Tm ( o. C) 41 41 47 48 49 58 58 66

Changing Asn to Other Amino Acids n Asn and Gln at high temperature Asn Asp + NH 3 Gln Glu + NH 3 ¨ Deamination becoming Asp and Glu ¨ Localized change in peptide folding loss of activity n S. cerevisiae triosephosphate isomerase (Table 8. 3) Two Asn residues in subunit interface Asn to Thr or Ile mutation increase in thermostability and protease resistance ¨ Asn to Asp mutation decrease in stability ¨ ¨ n Long lasting human insulin ¨ Mutation of an Asp to Gly

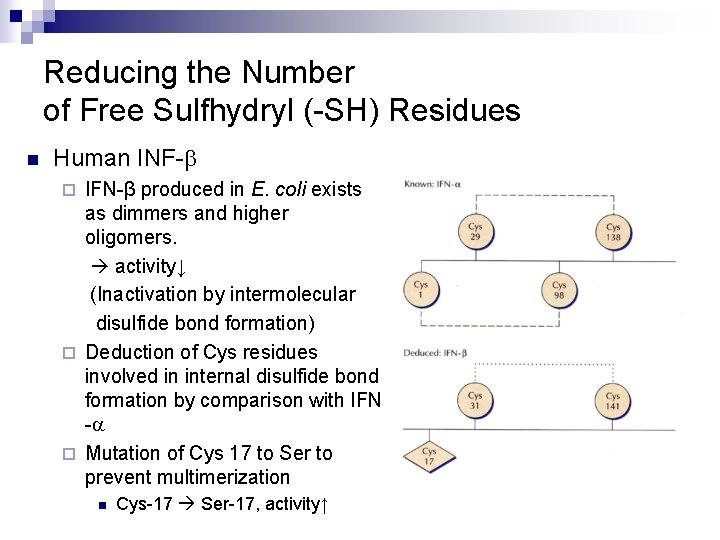
Reducing the Number of Free Sulfhydryl (-SH) Residues n Human INF-b IFN-β produced in E. coli exists as dimmers and higher oligomers. activity↓ (Inactivation by intermolecular disulfide bond formation) ¨ Deduction of Cys residues involved in internal disulfide bond formation by comparison with IFN -a ¨ Mutation of Cys 17 to Ser to prevent multimerization ¨ n Cys-17 Ser-17, activity↑

Increasing Enzyme Activity n n Change of one or more amino acid residues of active site activity↑ Change of Thr-51 to Ala-51 or Pro-51 (Table 8. 4) ¨ kcat/Km↑ ¨ kcat: catalytic rate constant ¨ Km: binding constant (Km↓, binding affinity↑)

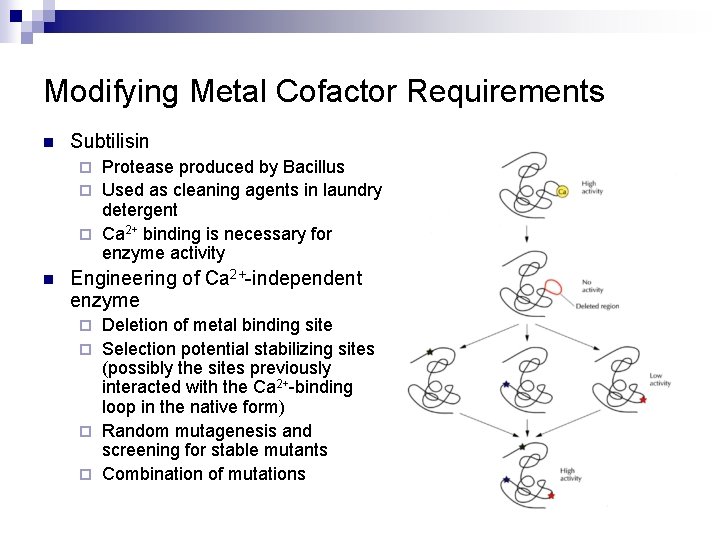
Modifying Metal Cofactor Requirements n Subtilisin Protease produced by Bacillus ¨ Used as cleaning agents in laundry detergent ¨ Ca 2+ binding is necessary for enzyme activity ¨ n Engineering of Ca 2+-independent enzyme Deletion of metal binding site ¨ Selection potential stabilizing sites (possibly the sites previously interacted with the Ca 2+-binding loop in the native form) ¨ Random mutagenesis and screening for stable mutants ¨ Combination of mutations ¨

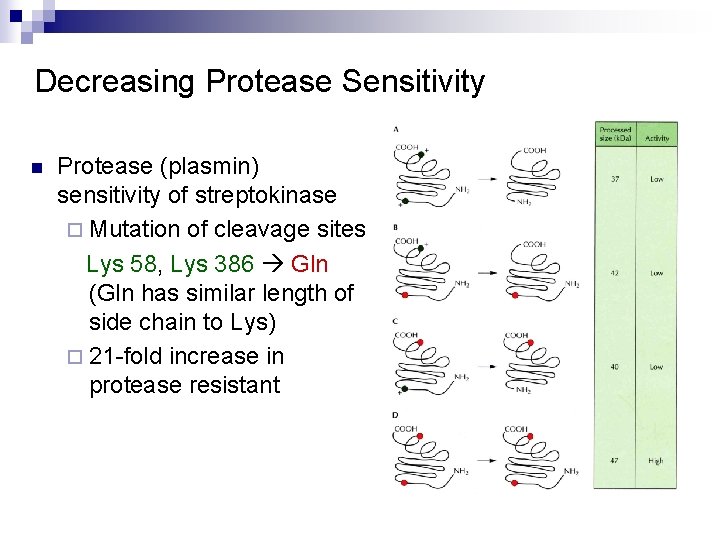
Decreasing Protease Sensitivity n Protease (plasmin) sensitivity of streptokinase ¨ Mutation of cleavage sites Lys 58, Lys 386 Gln (Gln has similar length of side chain to Lys) ¨ 21 -fold increase in protease resistant

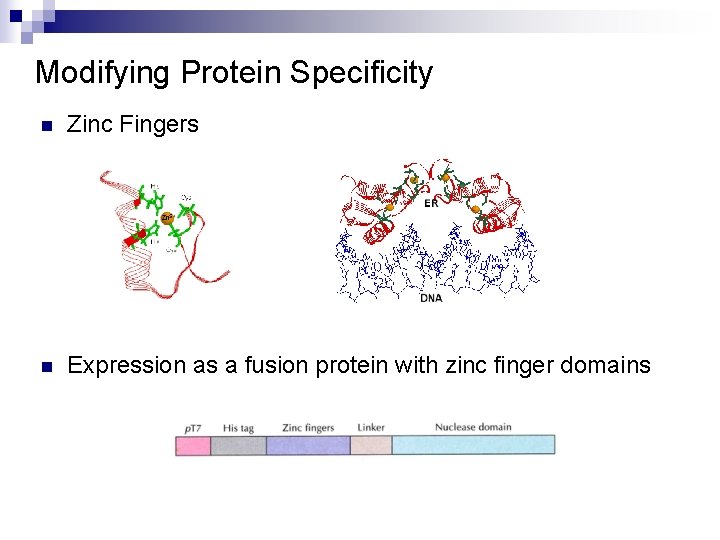
Modifying Protein Specificity n Restriction endonuclease >2, 500 enzymes but ~200 recognition sites ¨ Rare cutters are necessary for producing large DNA fragments ¨ n Protein engineering of Fok. I endonulcease with zinc finger Fok. I : nonspecific nuclease ¨ Zinc finger: ¨ n n ¨ Each zinc finger interacts with a specific DNA triplet codon. Using a combination of zinc fingers, they will bind to a predetermined site on a DNA fragment. Expression as a fusion protein with zinc finger domains n To create unique endonucleases

Modifying Protein Specificity n Zinc Fingers n Expression as a fusion protein with zinc finger domains

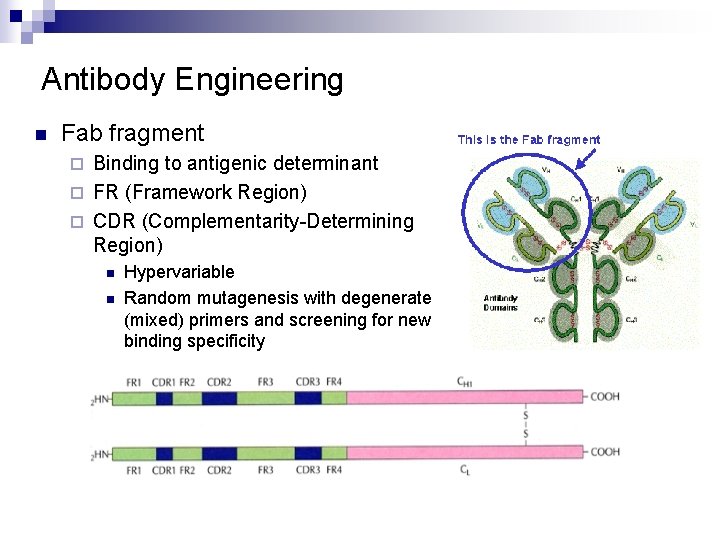
Antibody Engineering n Fab fragment Binding to antigenic determinant ¨ FR (Framework Region) ¨ CDR (Complementarity-Determining Region) ¨ n n Hypervariable Random mutagenesis with degenerate (mixed) primers and screening for new binding specificity

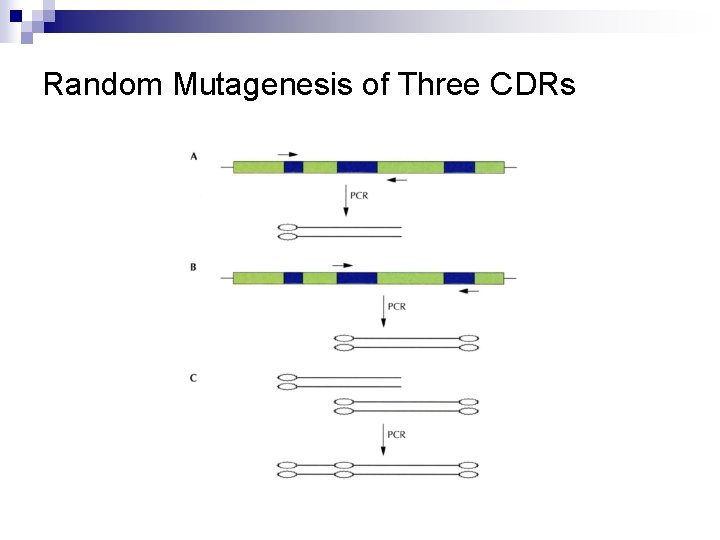
Random Mutagenesis of Three CDRs

Increasing Enzyme Stability and Specificity n Tissue plasminogen activator (t. PA) ¨ Serine protease ¨ Dissolution of blood clots n Protein engineering of t. PA ¨ Increase n Thr 103 to Asn ¨ Increase n in stability in fibrin binding Lys His Arg (296 -299) to Ala Ala

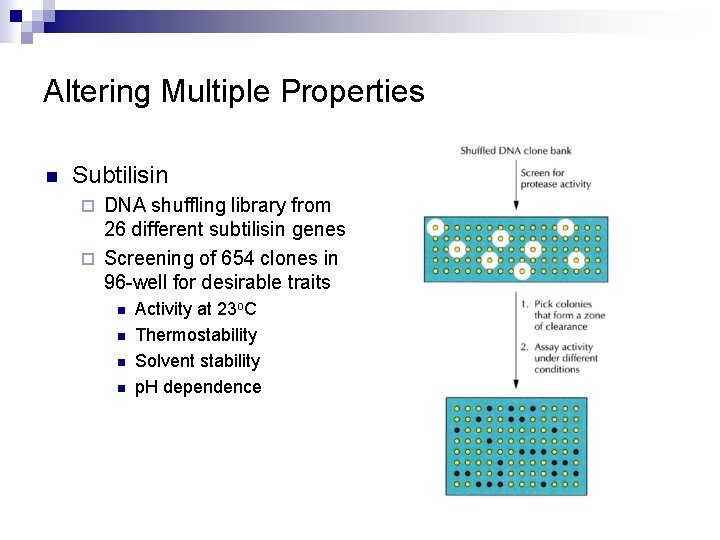
Altering Multiple Properties n Subtilisin DNA shuffling library from 26 different subtilisin genes ¨ Screening of 654 clones in 96 -well for desirable traits ¨ n n Activity at 23 o. C Thermostability Solvent stability p. H dependence

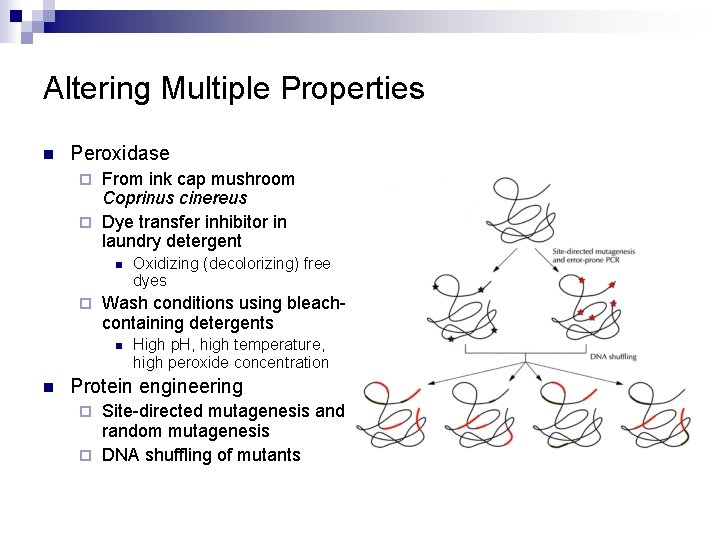
Altering Multiple Properties n Peroxidase From ink cap mushroom Coprinus cinereus ¨ Dye transfer inhibitor in laundry detergent ¨ n ¨ Wash conditions using bleachcontaining detergents n n Oxidizing (decolorizing) free dyes High p. H, high temperature, high peroxide concentration Protein engineering Site-directed mutagenesis and random mutagenesis ¨ DNA shuffling of mutants ¨