Prostate Artery Embolization Sandeep Bagla MD Cardiovascular Interventional

+ Prostate Artery Embolization Sandeep Bagla, MD Cardiovascular & Interventional Radiology Inova Alexandria Hospital Alexandria, Virginia USA

+ PAE - US Experience n Starting a PAE program n Prospective US Trial Experience n Safety n Efficacy n How do we perform the PAE procedure with Embozene? n Techniques to improve Safety n CBCT n Future Challenges n Questions

+ Disclosures n No significant financial disclosures or conflicts of interest (US PHS policy on FCOI, 2013) n Embolic agents discussed are FDA cleared (510 K clearance) for use in hypervascular tumors and vascular malformations. Other use is considered ‘off label’ n All cases presented are subjects enrolled in an IRB approved prospective research study n Prospective Pilot Study of Prostatic Artery Embolization in the Treatment of Benign Prostatic Hyperplasia. PI: Sandeep Bagla, MD. Study

+ Starting a PAE Program

+ All eyes on you

+ Competitive Environment Avoid the race to the top

+ Keys to a Successful Program What can IR do first? n Understand n The clinical evaluation of the BPH patient n Overlapping syndromes & Misdiagnoses Tests used in evaluating BPH and their As and Ds. n n Knowledge n n n the pathophysiology of BPH of treatment options How each procedure is performed Contraindications for certain treatments Limitations of different technologies Side Effects and complication rates of each procedure AUA Guidelines for BPH (2010)
+ Collaboration An Act of Shared Creation or Discovery

+ Building a Treatment Team Collaboration for Common Goal n Good clinical outcomes n Lower rate of complications n Treatment of patients who are not otherwise candidates for standard procedures n Generate Discovery and Innovation through Research n Collect Data n Produce Results n Publish Experience & Generate Practice Guidelines

+ Purpose n Evaluate the safety of Prostate Artery Embolization n Assess the efficacy in terms of clinical improvement in AUA symptom score n Measure the impact on Qo. L n Assess for changes in sexual function n Monitor for changes in urine flow rate and prostate volume

+ Study Design & Methodology n Prospective phase II/III trial nonrandomized treatment trial n Target enrollment is 30 patients n Statistically powered to show a mean difference of 7 points with a 0. 05 two sided level of significance n Study duration: 2 years after procedure

+ Study Design & Methodology Inclusion Criteria • Age > 50 • Moderate or Severe Symptoms, with AUA symptom score > 8 • LUTS felt to be related to BPH • Failed or refused medical or surgical therapy

+ AUA Symptom Score and Qo. L Voiding & Storage Symptoms • Mild: 1 -7 • Moderate: 8 -19 • Severe: 20 -35

+ Study Design & Methodology Exclusion Criteria • • Neurogenic bladder Creatinine > 1. 6 Uncorrectable coagualopathy Known prostate cancer Active bladder cancer < 2 yrs PSA > 4 unless negative bx Active Urinary Retention Active Urinary Infection
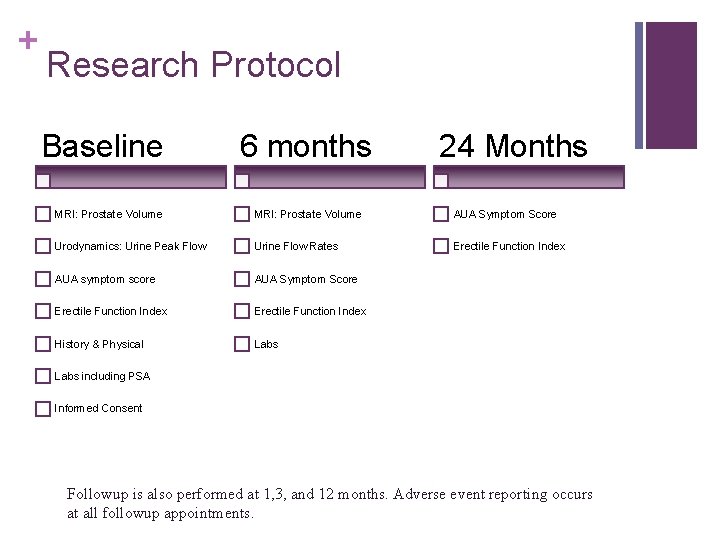
+ Research Protocol Baseline 6 months 24 Months MRI: Prostate Volume AUA Symptom Score Urodynamics: Urine Peak Flow Urine Flow Rates Erectile Function Index AUA symptom score AUA Symptom Score Erectile Function Index History & Physical Labs including PSA Informed Consent Followup is also performed at 1, 3, and 12 months. Adverse event reporting occurs at all followup appointments.
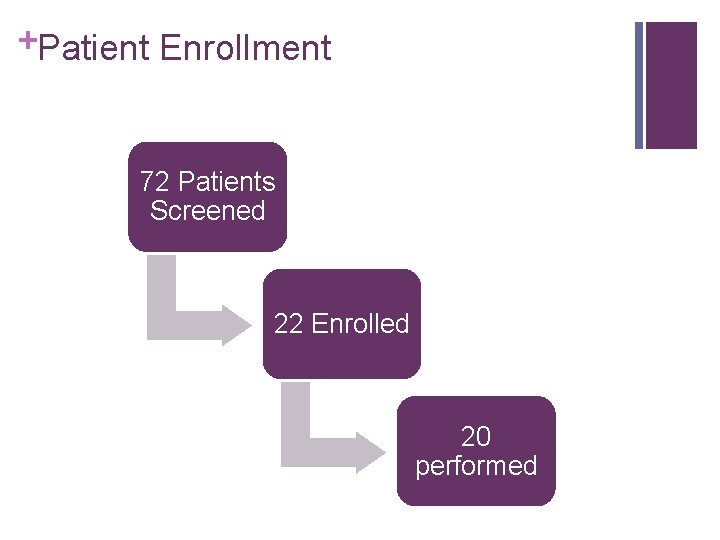
+Patient Enrollment 72 Patients Screened 22 Enrolled 20 performed
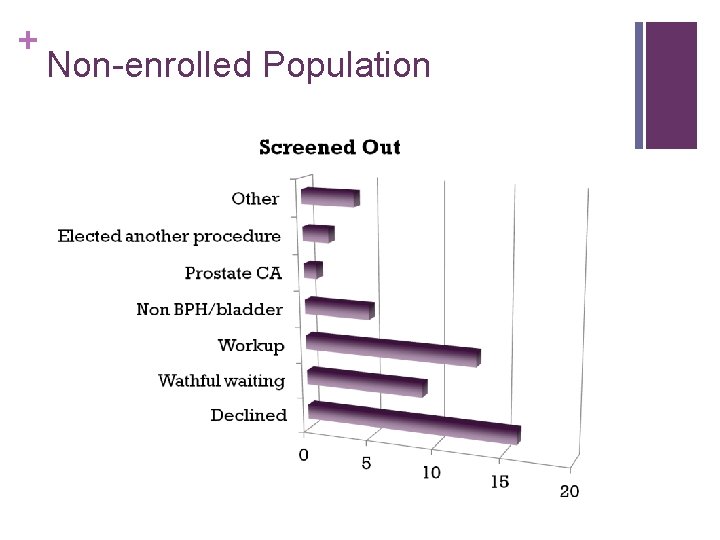
+ Non-enrolled Population
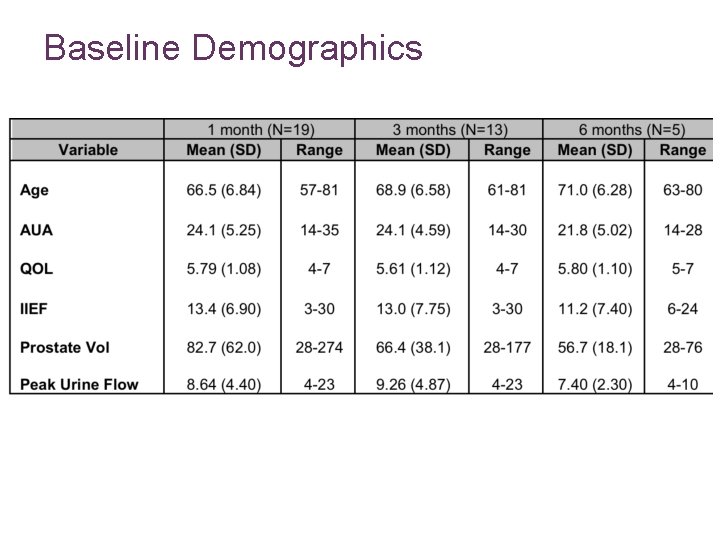
Baseline Demographics

+ Methods n Unilateral Femoral Access n Microcatheter selection of prostatic arteries n CBCT during parenchymal perfusion phase was performed to assess for non-target embolization n Embolization performed with spherical embolic agent (Embozene, Celonova) Range 100 -400 um n Injected in 0. 25 -0. 50 cc aliquots with angiography performed intermittently to assess for adequate antegrade flow. n Target end point: near stasis n Patients discharged same day: n Toradol on Proc Day, Motrin x 3 days n Cipro x 5 days n Pyridium TID x 2 days
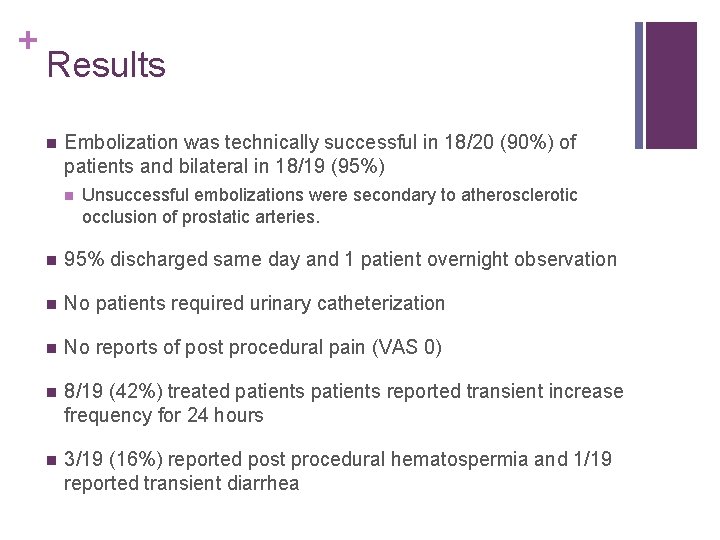
+ Results n Embolization was technically successful in 18/20 (90%) of patients and bilateral in 18/19 (95%) n Unsuccessful embolizations were secondary to atherosclerotic occlusion of prostatic arteries. n 95% discharged same day and 1 patient overnight observation n No patients required urinary catheterization n No reports of post procedural pain (VAS 0) n 8/19 (42%) treated patients reported transient increase frequency for 24 hours n 3/19 (16%) reported post procedural hematospermia and 1/19 reported transient diarrhea
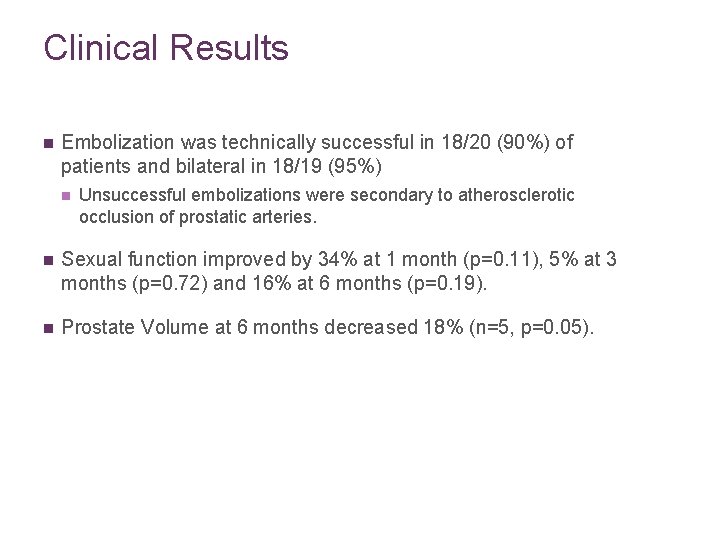
Clinical Results n Embolization was technically successful in 18/20 (90%) of patients and bilateral in 18/19 (95%) n Unsuccessful embolizations were secondary to atherosclerotic occlusion of prostatic arteries. n Sexual function improved by 34% at 1 month (p=0. 11), 5% at 3 months (p=0. 72) and 16% at 6 months (p=0. 19). n Prostate Volume at 6 months decreased 18% (n=5, p=0. 05).
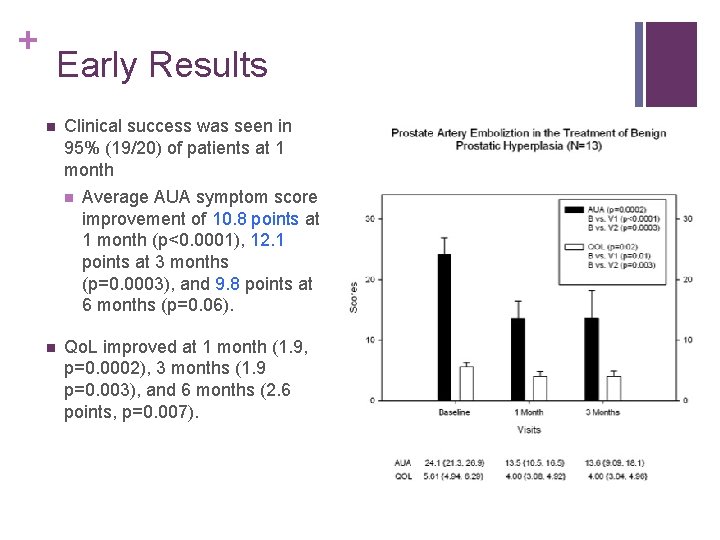
+ Early Results n Clinical success was seen in 95% (19/20) of patients at 1 month n n Average AUA symptom score improvement of 10. 8 points at 1 month (p<0. 0001), 12. 1 points at 3 months (p=0. 0003), and 9. 8 points at 6 months (p=0. 06). Qo. L improved at 1 month (1. 9, p=0. 0002), 3 months (1. 9 p=0. 003), and 6 months (2. 6 points, p=0. 007).

+ Procedural Results n Mean fluoroscopy time was 30. 2 mins (range 11. 5 -63. 9, 10 frames/sec) n Average dose 55923 μGycm 2 (range 5689 -339676). n Average procedure time was 72 minutes (range 41 -177 minutes).

+ Safety Results n No minor or major complications (SIR classification system for reporting) n No urologic complications including impotence, incontinence, prostatitis or non target embolization.

+ Other Endpoints & Outcomes n Prostate Volume Reduction n Peak Urine Flow Rate n Sexual Function n Cost n Radiation Exposure

+ Early Trial Results: Conclusion n Early results from this US trial demonstrate that PAE is a safe procedure and has short term efficacy. n Awaiting long terms results from the trial n Results parallel European and SA clinical results n Future trial at our institution will compare PAE to other minimally invasive BPH therapies
+ Technique Prostate Artery Embolization Sandeep Bagla, MD Inova Alexandria Hospital Alexandria, Virignia USA
+ CBCT 8 S DR/Siemens

+ Embozene® Microspheres

+ Delivering Embolic

+ Embozene Size Selection Target artery & Collateral Supply n Embozene 100 um n Embozene 250 um n Embozene 400 um

+ PROSTATIC ARTERIAL ANATOMY AND EMBOLIZATION WHAT DO WE NEED TO KNOW? HUGO RIO TINTO, MD 1. Hospital de Saint Louis 2. Hepatobiliary and Transplant Centre, Curry Cabral Hospital 3. Anatomy and Radiology Departments New University of Lisbon Portugal
+ III. Technical Obstacles n Spasm n Dissection n Artery Dimension n Extreme vascular tortuosity n End-Point n Non-target embolization n Impossible to catheterize
+ III. Technical Obstacles n Selective embolization n Catheter: Roberts Uterine 5 F n Microcatheter n n 2. 7 F to 2. 0 F n Shaped guidewire 0. 018’ Embolization Material n Embozene® n Non target embolization n n (250/400) Use micro-coils to avoid non-prostatic embolization End Point n Near stasis in prostatic vessels, arterial flow interruption and prostatic opacification

+ III. Technical Obstacles n To reach the prostatic micro-vascularization we should use small sized Embozene (250400) n The use of such small particles may cause non target embolization through anastomosis (>50%) n So, if anastomosis through capsular branches, we should upsize Embozene particles

+ III. Technical Obstacles Sometimes: We cannot enter easily in the internal iliac arteries due to tortuosity and atherosclerotic (old patients) We cannot enter the prostatic vessels We have big anastomoses to internal pudendal, rectal or vesical arteries!

+ IV. Inclusion Criteria n Age > 50 years. n Prostate > 40 g n Diagnosis of BPH with moderate to severe lower urinary tract symptoms (LUTS) refractory to medical treatment for at least 6 months; n With sexual dysfunction or accepting the risk of developing sexual dysfunction after treatment; n And one of the following: n IPSS > 18 and/or Qo. L> 3 and/or Qmax < 12 m. L/s and/or Acute urinary retention n Patients that cannot have general anaesthesia or have prostate dimension not suitable for TURP
+ IV. Exclusion Criteria n Advanced atherosclerosis and tortuosity of iliac arteries in Angio CT (exclusion rate < 5%) n Non visualization at all of prostatic arteries on Angio CT. n Malignancy (PSA, TRUS, Biopsy) n Detrusor Failure n Neurogenic Bladder n Bladder diverticulum or stones (surgery indicated) n Urethral stenosis
+ V. Complications/Follow-Up

+ V. Complications n Although most of the patients do not feel pain… …when they have be aware of possible complications… Addressed by ambulatory medical treatment (minor) Hospitalization or readmission; need for surgery (major)

+ V. Complications n Puncture site, contrast agents or radiation n Pelvic infection n Ischemic complications – non-prostatic embolization. n Sexual dysfunction. n Adverse drug reactions. .

+ V. Follow-Up n During n and 6 hours after PAE Questionnaires with a visual scale (0 -10) n Together n Clinical with Urology team; and Imaging.
+ VI. Results Clinical history and examination Severity of low urinary tract symptoms with IPSS Quality of life (Qo. L) Erectile/sexual function Uroflowmetry Digital examination Trans-rectal ultrasound and PSA MRI +Minor vs major complication (bladder/rectal ischaemia)
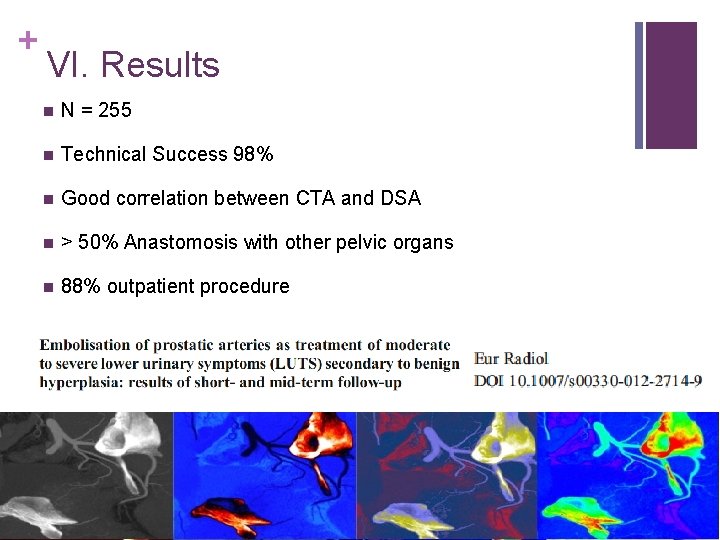
+ VI. Results n N = 255 n Technical Success 98% n Good correlation between CTA and DSA n > 50% Anastomosis with other pelvic organs n 88% outpatient procedure
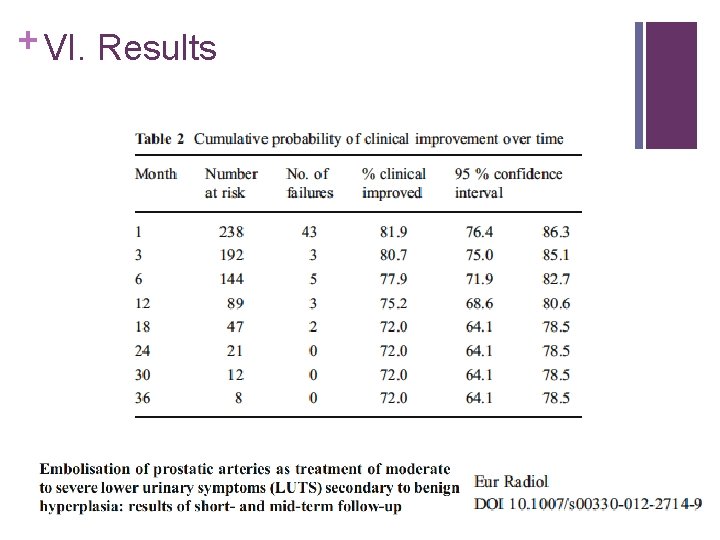
+ VI. Results

+ VII. Conclusions n Prostatic arterial embolization for benign prostatic hyperplasia is a promising future interventional radiology technique; n It is essential to know the most relevant anatomical features to avoid complications;

+ VII. Conclusions n Difficult anatomy due to several variations; n Tortuosity n Small and atherosclerosis limits feasibility; anastomosis may go undetected with conventional angiography; n No significant post-embolization symptoms, although potential serious complications due to non-target embolization;

+ VII. Conclusions n Suspect ischemic complications if severe pain develops; n Should be performed by someone/group with experience to avoid complications; n Suitable follow-up with both IR and Urology.

+ VII. Conclusions This can be a long and difficult procedure, so do not give up easily… If you want to go fast, go alone If you want to go far, go together

+ Thank You Hugo Rio Tinto hugo. tinto@gmail. c
- Slides: 50