Propofol Diprivan and Its Adverse Effect in Anesthesia



















- Slides: 27

Propofol (Diprivan) and It’s Adverse Effect in Anesthesia Liu, Chih-Min 2003 -8 -19

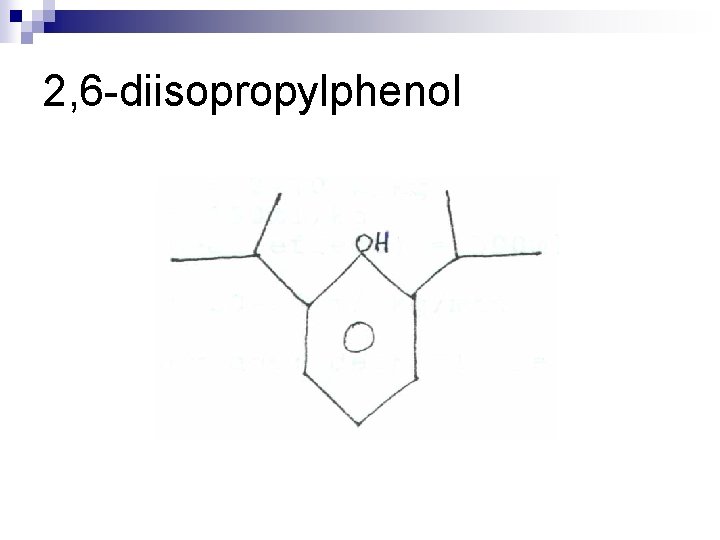
2, 6 -diisopropylphenol

Propofol Indications: Anesthesia, general n Pregnancy Category B n FDA Approved 1989 Oct n 2, 6 -diisopropylphenol n molecular weight of 178. 27. n isotonic n p. H of 7 -8. 5 n

Mechanism of Action The actual mechanism of action is unknown, but it is postulated that propofol mediates activity of the GABA receptors. n rapid sedation with minimal excitatory activity n no analgesic properties n

Pharmacokinetics n n n eliminated by hepatic conjugation to inactive metabolites, excreted by the kidney No dosage adjustments are needed for patients with renal or hepatic failure Geriatrics ¨ age-related decrease in volume of distribution ¨ higher peak plasma concentrations ¨ cardiorespiratory effects including hypotension, apnea, airway obstruction, and/or oxygen desaturation

INDICATIONS AND USAGE: induction and/or maintenance of anesthesia n in adult patients and pediatric patients greater than 3 years of age n not recommended for obstetrics n not recommended for use in nursing mothers n

Pediatric Use n not recommended in: ¨ induction of anesthesia in patients younger than 3 years of age ¨ maintenance of anesthesia in patients younger than 2 months of age ¨ not indicated for use in pediatric patients for ICU sedation

Cardiac Anesthesia n n n well-studied in coronary artery disease, but valvular or congenital heart disease is limited decrease in blood pressure that is secondary to decreases in preload and afterload lower heart rates possibly due to reduction of the sympathetic activity and/or resetting of the baroreceptor reflexes anticholinergic agents should be administered when increases in vagal tone are anticipated

Induction of General Anesthesia n n n Healthy Adults Less Than 55 Years of Age: 40 mg every 10 seconds until induction onset (2 -2. 5 mg/kg). Elderly, Debilitated, or ASA III/IV Patients: 20 mg every 10 seconds until induction onset (1 -1. 5 mg/kg). Cardiac Anesthesia: 20 mg every 10 seconds until induction onset (0. 5 -1. 5 mg/kg). Neurosurgical Patients: 20 mg every 10 seconds until induction onset (1 -2 mg/kg). Pediatric Patients - healthy, from 3 -16 years of age: 2. 5 -3. 5 mg/kg administered over 20 -30 seconds.

Maintenance of General Anesthesia n n n Healthy Adults Less Than 55 Years of Age: 100 -200 μg/kg/min (612 mg/kg/h). Elderly, Debilitated, ASA III/IV Patients: 50 -100 μg/kg/min (3 -6 mg/kg/h). Pediatric Patients - healthy, from 2 months to 16 years of age: 125 -300 μg/kg/min (7. 5 -18 mg/kg/h) Cardiac Anesthesia, Most Patients Require: Primary propofol injectable emulsion with secondary opioid: 100 -150 μg/kg/min. Lowdose propofol injectable emulsion with primary opioid: 50 -100 μg/kg/min. Neurosurgical Patients: 100 -200 μg/kg/min (6 -12 mg/kg/h).

CONTRAINDICATIONS n hypersensitivity to propofol injectable emulsion or its components, or when general anesthesia or sedation are contraindicated.

Beneficial Effects Sedation n Amnesia n

Adverse Effects n Airway ¨ Copious secretions ¨ Laryngospasm n Respiratory ¨ Apnea, respiratory depression ¨ Hiccough ¨ Bronchospasm n Cardiovascular ¨ Hypotension ¨ Dysrhythmias, bradycardia or tachycardia

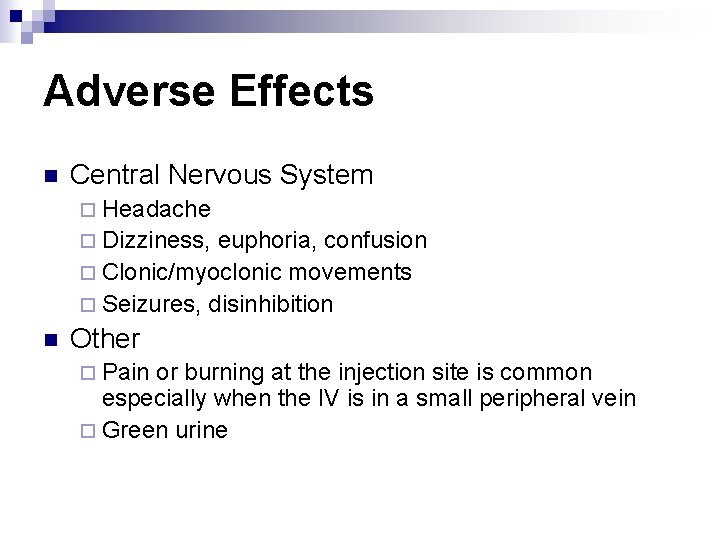
Adverse Effects n Central Nervous System ¨ Headache ¨ Dizziness, euphoria, confusion ¨ Clonic/myoclonic movements ¨ Seizures, disinhibition n Other ¨ Pain or burning at the injection site is common especially when the IV is in a small peripheral vein ¨ Green urine

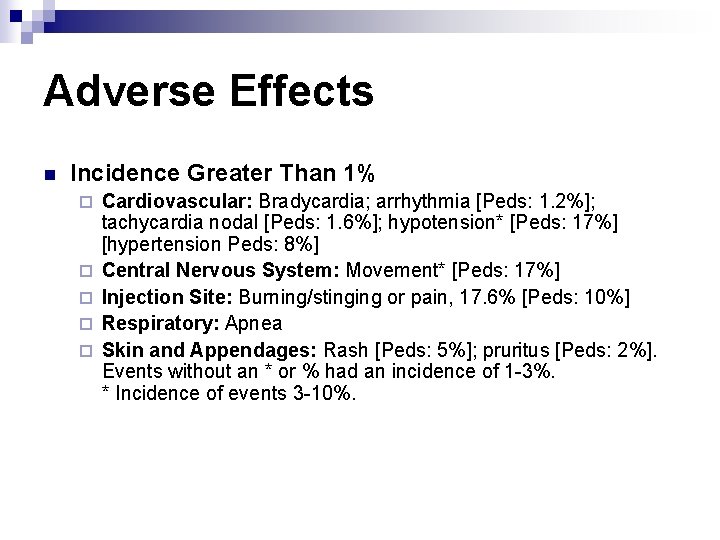
Adverse Effects n Incidence Greater Than 1% ¨ ¨ ¨ Cardiovascular: Bradycardia; arrhythmia [Peds: 1. 2%]; tachycardia nodal [Peds: 1. 6%]; hypotension* [Peds: 17%] [hypertension Peds: 8%] Central Nervous System: Movement* [Peds: 17%] Injection Site: Burning/stinging or pain, 17. 6% [Peds: 10%] Respiratory: Apnea Skin and Appendages: Rash [Peds: 5%]; pruritus [Peds: 2%]. Events without an * or % had an incidence of 1 -3%. * Incidence of events 3 -10%.

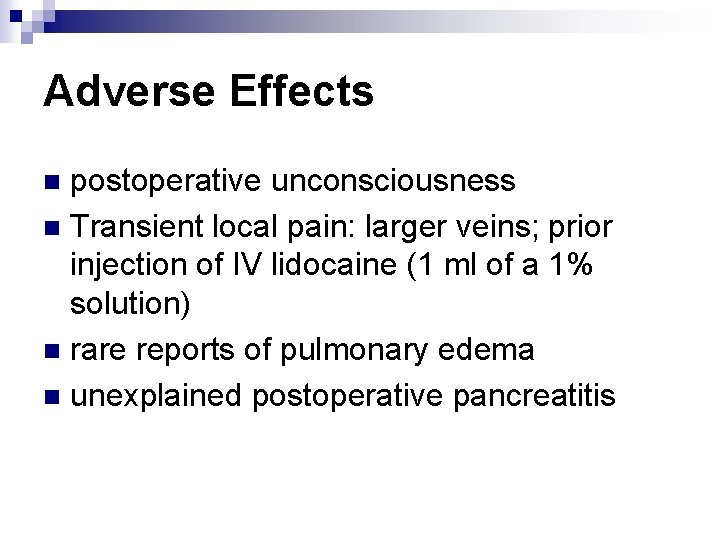
Adverse Effects postoperative unconsciousness n Transient local pain: larger veins; prior injection of IV lidocaine (1 ml of a 1% solution) n rare reports of pulmonary edema n unexplained postoperative pancreatitis n

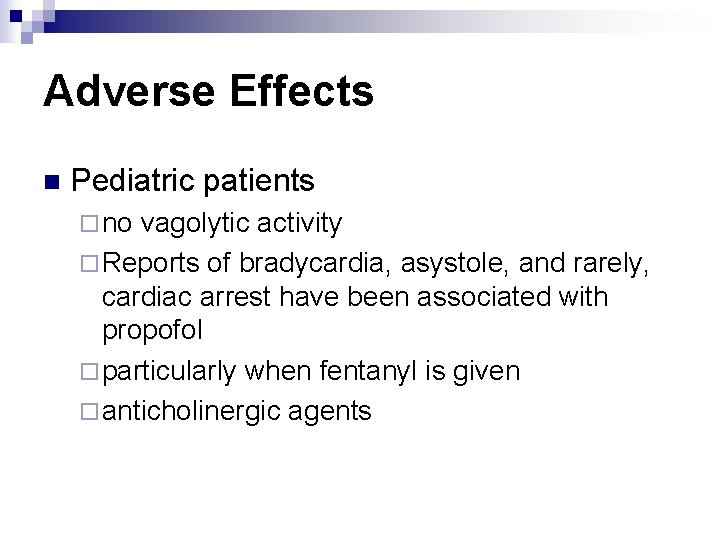
Adverse Effects n Pediatric patients ¨ no vagolytic activity ¨ Reports of bradycardia, asystole, and rarely, cardiac arrest have been associated with propofol ¨ particularly when fentanyl is given ¨ anticholinergic agents

DRUG INTERACTIONS: n dose requirements redused: ¨ Premedication with narcotics (e. g. , morphine, meperidine, and fentanyl, etc. ) ¨ In pediatric patients, administration of fentanyl concomitantly with propofol may result in serious bradycardia ¨ combinations of opioids and sedatives (e. g. , benzodiazepines, barbiturates, chloral hydrate, droperidol, etc. ) ¨ more pronounced decreases in systolic, diastolic, and mean arterial pressures and cardiac output

DRUG INTERACTIONS: ¨ reduced in the presence nitrous oxide ¨ inhalational agents (e. g. , isoflurane, enflurane, and halothane) has not been extensively evaluated ¨ does not cause a clinically significant change in onset, intensity or duration of action of the commonly used neuromuscular blocking agents (e. g. , succinylcholine and nondepolarizing muscle relaxants).

A small dose of midazolam decreases the time to achieve hypnosis without delaying emergence during short-term propofol anesthesia. Journal of Clinical Anesthesia Volume 13 • Number 4 • June 2001 Copyright © 2001 Elsevier n CONCLUSIONS: ¨ Coadministration of 10 microg kg(-1)midazolam decreases the dose and time required to achieve hypnosis with propofol induction without delaying emergence from anesthesia. ¨ Additional administration of flumazenil further shortens the time to emerge from midazolam-propofol anesthesia.

Seizure-like phenomena and propofol: a systematic review. Neurology Volume 58 • Number 9 • May 14, 2002 Copyright © 2002 American Academy of Neurology a change in cerebral concentration of propofol may be causal n a drug-induced excitation of the CNS, [9] including seizures in susceptible patients n warned about the use of propofol in patients with epilepsy n

The interaction between fentanyl and propofol during emergence from anesthesia: monitoring with the EEGBispectral index. Journal of Clinical Anesthesia Volume 15 • Number 2 • March 2003 Copyright © 2003 Elsevier n CONCLUSIONS: The plasma levels of fentanyl affect the concentrations of propofol required for patients to regain consciousness. ¨ The BIS values for wakefulness are unaltered at the different combinations of propofol and fentanyl concentrations. Thus, the BIS appears to be a useful and consistent indicator for level of consciousness during emergence from propofol/fentanyl intravenous anesthesia ¨

Death related to propofol use in an adult patient. Critical Care Medicine Volume 28 • Number 8 • August 2000 Copyright © 2000 Lippincott Williams & Wilkins ¨ several reports linking propofol to the development of metabolic acidosis and cardiac dysrhythmias in pediatric patients ¨ Arrhythmias, metabolic acidosis, cardiac failure, and death related to propofol use can occur in adults as well as in children

Metabolic acidosis and fatal myocardial failure after propofol infusion in children: five case reports BMJ 1992; 305: 613– 616 ¨ increasing metabolic acidosis was associated with brady-arrhythmia and progressive myocardial failure, which did not respond to resuscitative measures ¨ CONCLUSION— n Although the exact cause of death in these children could not be defined, propofol may have been a contributing factor.

Morphologic changes in the upper airway of children during awakening from propofol administration. Anesthesiology Volume 96 • Number 3 • March 2002 Copyright © 2002 American Society of Anesthesiologists, Inc. n CONCLUSIONS: The dimensions of the upper airways of children change shape significantly on awakening from propofol sedation. ¨ When sedated, the upper airway is oblong shaped, with the A-P diameter larger than the transverse diameter. ¨ On awakening, the shape of the upper airway in most children changed such that the transverse diameter was larger. Crosssectional areas between sedated and awakening states were unchanged. ¨ These changes may reflect the differential effects of propofol on upper airway musculature during awakening ¨

A comparison of ketamine and lidocaine spray with propofol for the insertion of laryngeal mask airway in children: a double-blinded randomized trial. Anesth Analg - 01 -DEC-2002; 95(6): 1586 -9 ¨ Ketamine and lidocaine spray appear to be appropriate for laryngeal mask airway (LMA) insertion in children. ¨ apnea and airway obstruction, the two most serious and frequent complications of propofol, can be avoided during LMA insertion.

Thanks for your attention!