PROPIONIC ACIDEMIA MELISSA ALEX DISEASE OVERVIEW Propionic acidemia










- Slides: 13

PROPIONIC ACIDEMIA MELISSA ALEX

DISEASE OVERVIEW Propionic acidemia (PPA) is an organic academia caused by mutations in the propionyl-Co. A carboxylase (PCC) gene – mitochondrial biotin dependent enzyme – catabolism of valine, isoleucine, methionine, threonine, CHL, odd chain FA • Propionyl Co. A → methylmalonyl Co. A • Block in propionyl PW – accumulation organic acids • Blood, cerebrospinal fluid, urine • Results – toxic manifestations • acidosis, neurological disease • Enzyme deficiency – generation of significant derangements • Intracellular MTB • Mitochondrial MTB Organic acidemia (OA) – subset AA disorders • Deficiency – PCC activity • Not a dysfunction of AA MTB • Intermediary product of MTB • Affected AA – val, ile, met, thr

ETIOLOGY Classification • Inherited • Autosomal recessive • Organic acid disorder • Reported in families of Amish ancestry • 1: 80, 000 1961 • characterized by ↑ serum glycine level (now indicates multiple enzyme deficiency disorders) suspected disorder of AA MTB w/ ↑ serum and urine propionate 1969 • peripheral blood leukocytes demonstrated deficiency of PCC activity PCC dodecamer (12 subunits- tetrahedral) • Chromosome 13 q 22 -q 34 • 6 β subunits • 6 biotin - containing α subunits • Defects within both subunits

SYMPTOMOLOGY • Presents 1 st few days to weeks of life – rare late adult onset • Hx family disease /unexplained neonatal death/acidopathic sibling • Degree of disease symptoms – enzyme impairment d/t genetic lesion • Neonates • Neurological deterioration, feeding refusal, vomiting, wt. loss, hypotonia, abnormal posturing/movements, lethargy, seizures, coma, severe brain damage, or death within days • Late manifest • Immune and bone morrow suppression, recurrent infections, neurological sequlae, mental retardation • Pt previous dx – acute onset abnormal movement → signs infraction basil ganglia • Metabolic acidosis, ↑ plasma ammonia/propionic acid, ↑ urine methylcitric acid • Cardiomyopathy – ¼ pt starting at 10 months to adulthood • ↓ cardiac carnitine – serum carnitine WNL

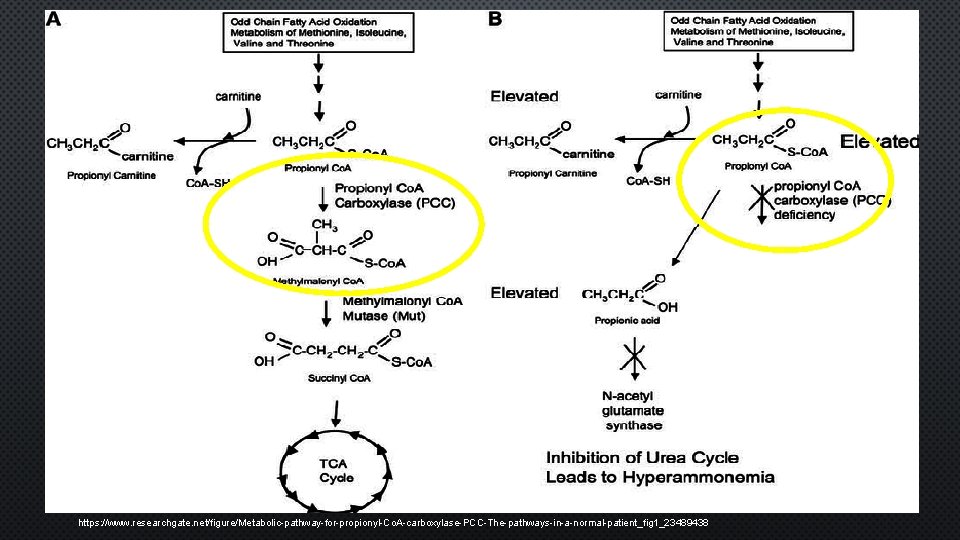
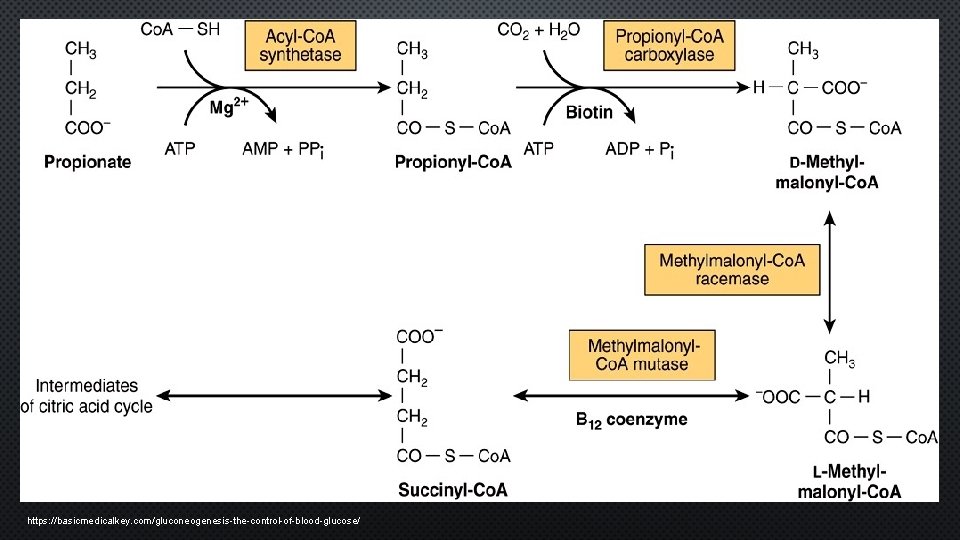
BIOCHEMICAL PATHWAY • Interruption of formation – energy producing intermediates of CAC • Major precursor glu ruminants • Gluconeogenesis – CAC • Esterification with Co. A • Catalyzed – Propionyl Co. A Carboxylase (PCC) • Non-ruminants • Propionate – B ox OCFA in ruminant lipids, oxidation isoleucine side chain of CHL, minor gluconeogenesis substrate • PCC – mitochondria/biotin containing enzyme • Defective BCAA & OCFA MTB → propinoyl accumulation • Defects PA → toxic metabolites ↑ propionic acid (brain/NS) • Dysfunction PW – toxic endothelial cells & basil ganglia

https: //www. researchgate. net/figure/Metabolic-pathway-for-propionyl-Co. A-carboxylase-PCC-The-pathways-in-a-normal-patient_fig 1_23489438

https: //basicmedicalkey. com/gluconeogenesis-the-control-of-blood-glucose/

NUTRITIONAL IMPLICATIONS • Metabolic crisis • Feeding changes 2º infections • Recurrent episodes • Metabolic decomposition d/t inadequate PO feeding • Illness w/fever endogenous catabolism • Dehydration • Gastrointestinal bacteria – produce propionic acid • Tx – metronidazole • Constipation • Essential FA deficiency • Feeding problems • Recurrent episodes

MEDICAL NUTRITION THERAPY • ↓ Pro foods – formula w/o ile, met, thr, val • Restrict AA (dietary precursors) –reduce toxic OA • Synthetic AA based formulas – 50% pro/d • Milder genotypes • Intact Pro – LBVP • Fruits, veggies, limited grains • Infant pro intake: 1 -1. 5 g Pro/kg • Dilute standard formula – add pro free formula • Formula – limits thr, ile & omits met, val • Moderate pro/high energy – CHO/FAT • Similar requirements PKU diet • Fluid – 64 oz. min • ↑ fluid needs – removal of abnormal metabolites

MEDICAL NUTRITION THERAPY • Avoid fasting – prevent muscle pro catabolism → OA build up • Frequent meals – q 2 -3 h • “Sick Day Diet” • Natural Pro intake ↓ 50% - ↑ non pro kcal • Small, frequent meals/snacks as tol • Formula supplement • Electrolyte drink, Drip Drop • Preventative Measurements • PEG – effective ↓ hospitalization & chronic management adequate nutrition • Nasogastric – acute relapse/feeding aversion • Goals • Maintenance energy/fluid intake • Biochemical balance – promote anabolism, normal growth/development • Prevent catabolism/dehydration • Prevent metabolic acidosis relapse – infection, high pro intake, constipation • ↑ fluid requirements – monitor electrolyte balance • Encourage PO intake • Collaboration – SLP

MONITORING/EVALUATION • Education • Early signs: dehydration, poor PO intake, formula/food preparation, diet recommendations, label reading & food choices • Labs • Alb, prealb, total pro, ammonia, plasma AA, Na, K, Cl, BUN, Cr, bicarbonate (CO 2), BGL, urine ketones, serum lactate, serum pyruvate, uric acids, carnitine • PO Intake • Pro/kcal • Chewing/swallowing/feeding problems, decrease appetite, feeding refusal • Hydration • Fluid intake - I/O • Ht. /Wt. • Essential FA deficiency, vitamins, minerals - NFPE • Illness relapse

ADJUNCT TREATMENT • Carnitine • Binds OA, Na benzoate/Na phenylbutyrate removes excess Nitrogen - ↓ ammonia production • Supplementation: 200 – 300 mg/kg bid/tid • Recurrent metabolic decomposition/hyperammonia (↑ range) • Laxative • ↓ serum ammonia, ↓ propionylglycine urine excretion • ↑ratio free total carnitine (protein motility) • Biotin • evidence conflicts - ↑ isoleucine MTB & ↓ propionate production • Bicarbonate • Acute episodes • IV fluids • Liver transplant – limit intellectual disability & cardiac damage • MVT

REFERENCES Sutton, V. R. , Chapman, K. A. , Gropman, A. L. , Mac. Leod, E. , Stagni, K. , Summar, M. L. , . . . & Matern, D. (2012). Chronic management and health supervision of individuals with propionic acidemia. Molecular genetics and metabolism, 105(1), 26 -33. Feliz, B. , Witt, D. R. , & Harris, B. T. (2003). Propionic acidemia: a neuropathology case report and review of prior cases. Archives of pathology & laboratory medicine, 127(8), e 325 -e 328. Nyhan, W. L. , Bay, C. , Beyer, E. W. , & Mazi, M. (1999). Neurologic nonmetabolic presentation of propionic acidemia. Archives of neurology, 56(9), 1143 -1147. Yannicelli, S. (2006). Nutrition therapy of organic acidaemias with amino acid-based formulas: emphasis on methylmalonic and propionic acidaemia. Journal of inherited metabolic disease, 29(2 -3), 281 -287. Lehnert, W. , Sperl, W. , Suormala, T. , & Baumgartner, E. R. (1994). Propionic acidaemia: clinical, biochemical and therapeutic aspects. European journal of pediatrics, 153(1), S 68 -S 80. De Baulny, H. O. , Benoist, J. F. , Rigal, O. , Touati, G. , Rabier, D. , & Saudubray, J. M. (2005). Methylmalonic and propionic acidaemias: management and outcome. Journal of inherited metabolic disease, 28(3), 415 -423. Chapman, K. A. , Gropman, A. , Mac. Leod, E. , Stagni, K. , Summar, M. L. , Ueda, K. , . . . & Pena, L. (2012). Acute management of propionic acidemia. Molecular genetics and metabolism, 105(1), 16 -25. Huang, C. S. , Sadre-Bazzaz, K. , Shen, Y. , Deng, B. , Zhou, Z. H. , & Tong, L. (2010). Crystal structure of the α 6 β 6 holoenzyme of propionyl-coenzyme A carboxylase. Nature, 466(7309), 1001. Gliksman, F. J. , & Lutsep, H. L. (2017, December 11). Propionic Acidemia. Retrieved January 20, 2018, from https: //emedicine. medscape. com/article/1161910 -overview Nelms, M. N. , Sucher, K. , Lacey, K. , Habash, D. , Nelms, G. R. , Hansen-Petrik, M. , . . . Wong, J. (2011). Nutrition therapy and pathophysiology (Second ed. ). Boston, MA: Cengage Learning. Mahan, L. K. , Escott-Stump, S. , Raymond, J. L. , & Krause, M. V. (2012). Krauses food & the nutrition care process (13 th ed. ). St. Louis, MO: Elsevier/Saunders.