Properties of Water TSW describe the properties of

Properties of Water TSW describe the properties of water that make it essential to life on Earth

What is water? Ø H 2 O…which means? 1 molecule of water has 2 atoms of Hydrogen & 1 atom of Oxygen Ø Water covers up to 75% of the Earth’s surface & makes up 70 -95% of all living cells!
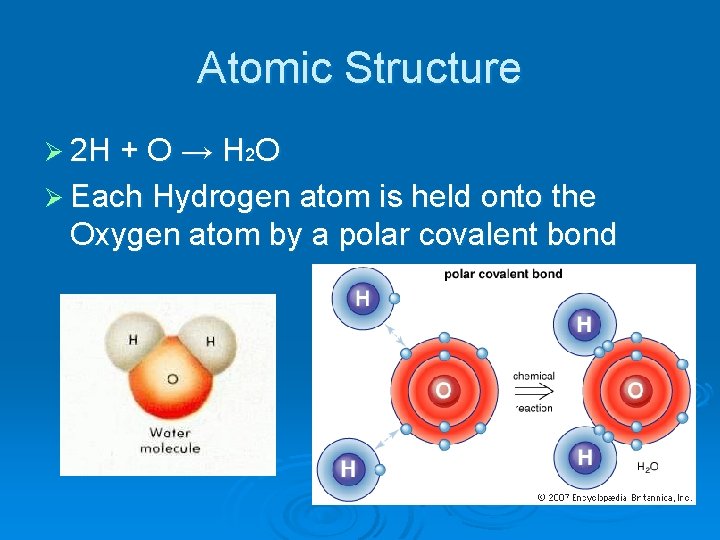
Atomic Structure Ø 2 H + O → H 2 O Ø Each Hydrogen atom is held onto the Oxygen atom by a polar covalent bond
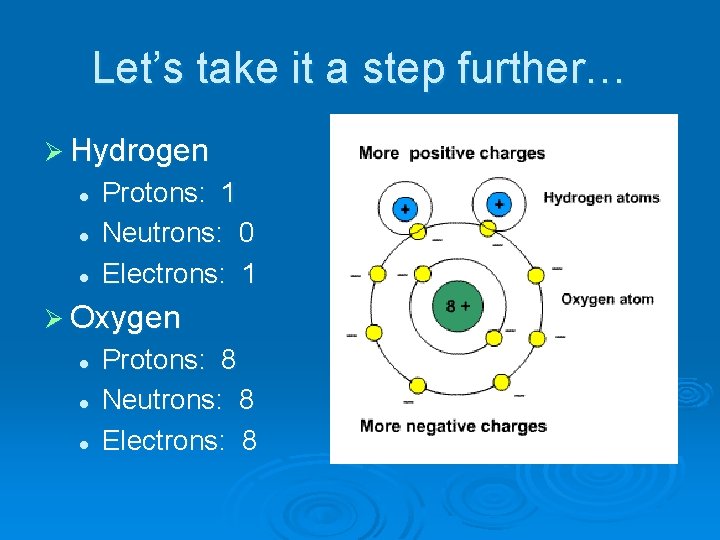
Let’s take it a step further… Ø Hydrogen l l l Protons: 1 Neutrons: 0 Electrons: 1 Ø Oxygen l l l Protons: 8 Neutrons: 8 Electrons: 8
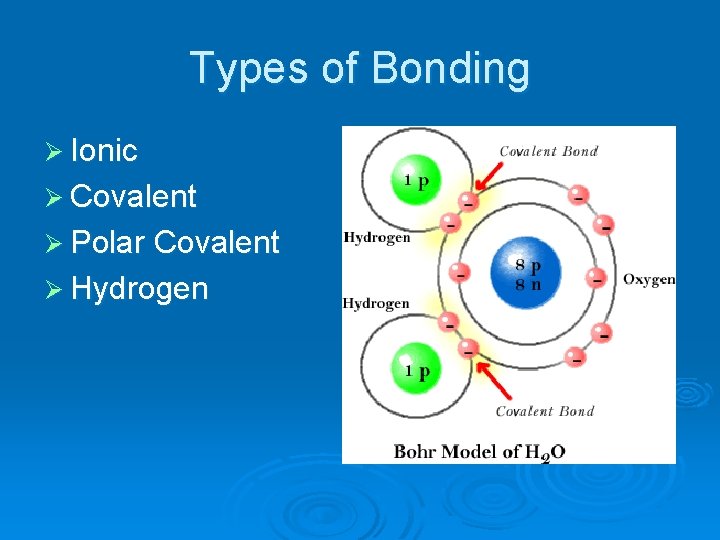
Types of Bonding Ø Ionic Ø Covalent Ø Polar Covalent Ø Hydrogen
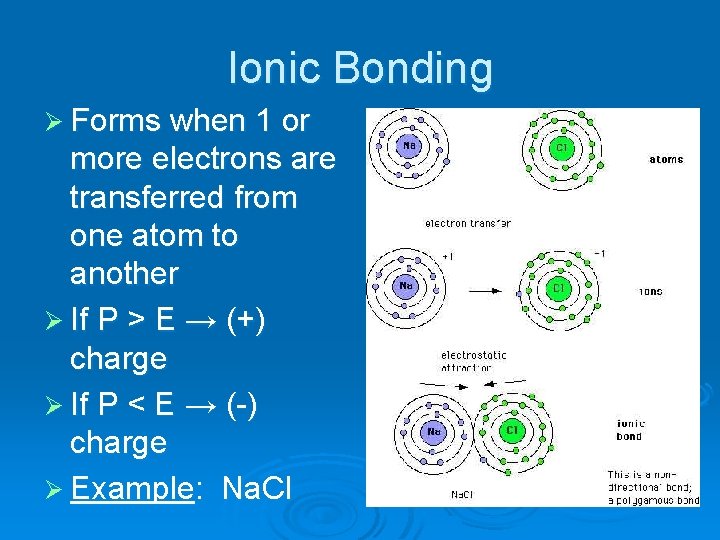
Ionic Bonding Ø Forms when 1 or more electrons are transferred from one atom to another Ø If P > E → (+) charge Ø If P < E → (-) charge Ø Example: Na. Cl
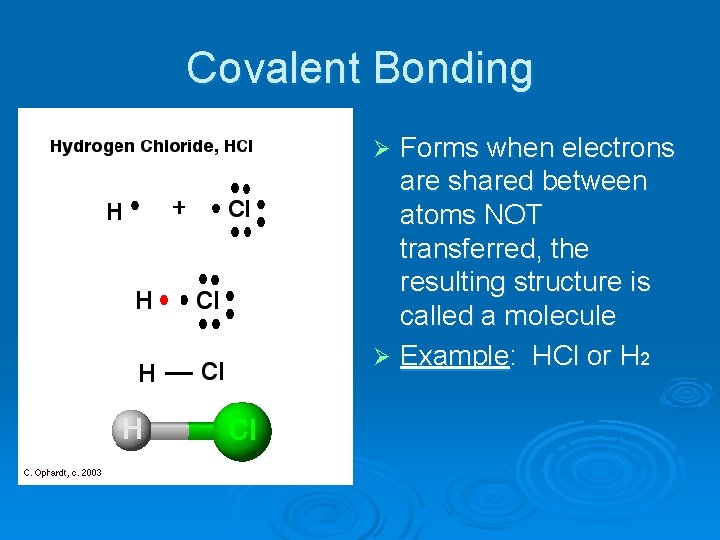
Covalent Bonding Forms when electrons are shared between atoms NOT transferred, the resulting structure is called a molecule Ø Example: HCl or H 2 Ø
Polar Covalent Bonding Ø Forms when sharing occurs in an unequal manner (the molecule formed has no net charge) Ø Opposite ends of the molecule are partially (-) & partially (+) depending on the distribution of electrons Ø Example: H 2 O
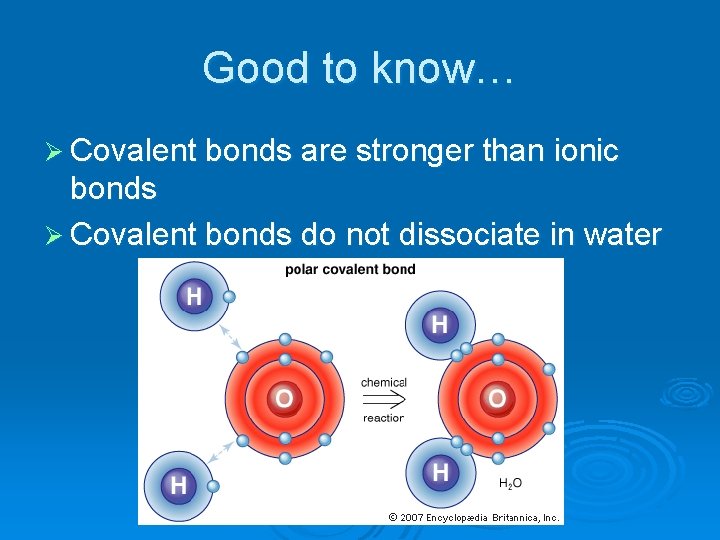
Good to know… Ø Covalent bonds are stronger than ionic bonds Ø Covalent bonds do not dissociate in water

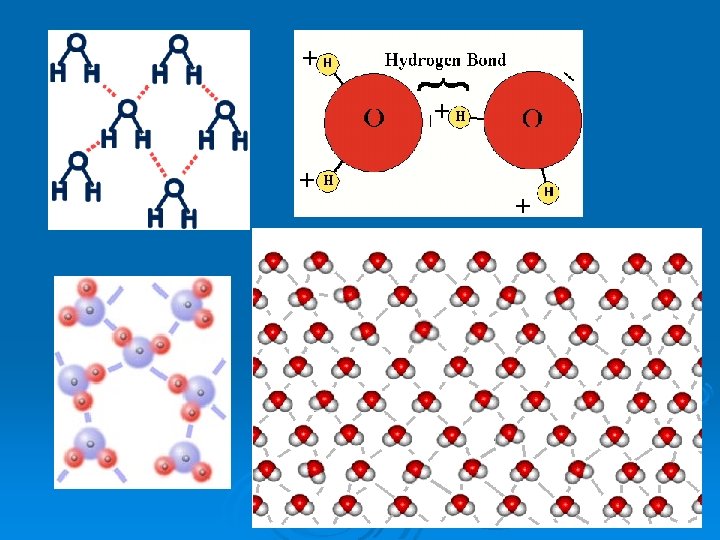
Hydrogen Bonding Ø Forms between water molecules Ø H+ bonds to OØ Easy to break Ø Key Point: The numerous special qualities of water are made possible by its ability to form multiple H bonds
- Slides: 11