Properties of Water The most important inorganic compound













- Slides: 18

Properties of Water The most important inorganic compound for living organisms!

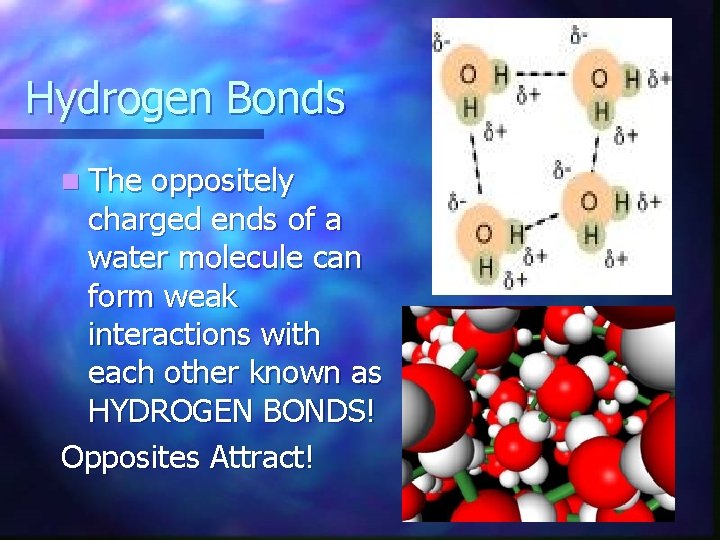
Water is POLAR! Water has an unequal distribution of charges…the O atom has a (-) charge & each H atom has a (+) charge. n Polarity refers to the property of having 2 opposite poles. n HOW DOES THIS RELATE?

Hydrogen Bonds n The oppositely charged ends of a water molecule can form weak interactions with each other known as HYDROGEN BONDS! Opposites Attract!

Cohesive n An attraction of molecules of the same substance n Water can “stick” to itself…. surface tension! n Explains why some insects can “walk on water”

Adhesive n An attraction between molecules of different substances. n Allows for capillary action…. draws water out of roots up into the stems.

Water has a high heat capacity…what does that mean? n Water is able to absorb large amounts of heat. n As a result, lakes and oceans stabilize air and land temperatures.

Ice floats? n Yes, Water is more dense as a liquid than a solid! n Prevents lakes & ponds from freezing solid…life can survive at the bottom n Also allows for mixing of nutrients in water

Universal Solvent n Most all of our chemical reactions take place in SOLUTIONS…. comprised of a solute (the substance dissolved) and a solvent (the substance in which the solute dissolves)…water is the greatest solvent on Earth!

In summation, n Water is essential for life on Earth!

p. H and the Importance to Life

What exactly is p. H? Well, it stands for the potential of hydrogens, but simply put…. n It is a measure of the concentration of H+ ions in a solution n

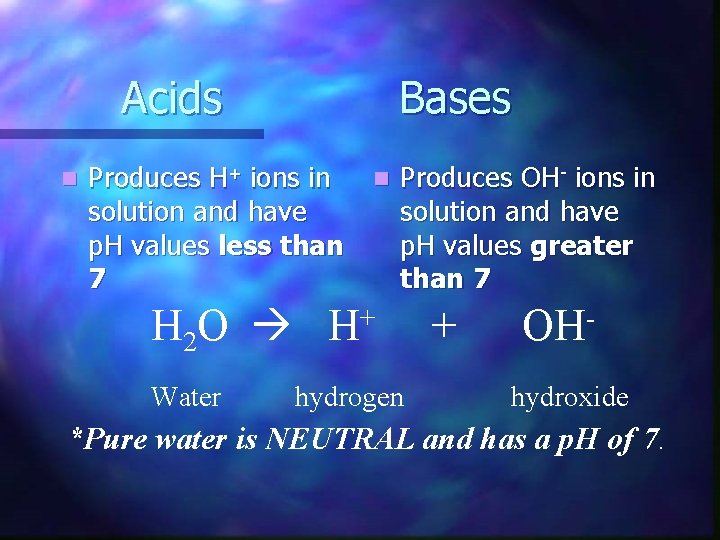
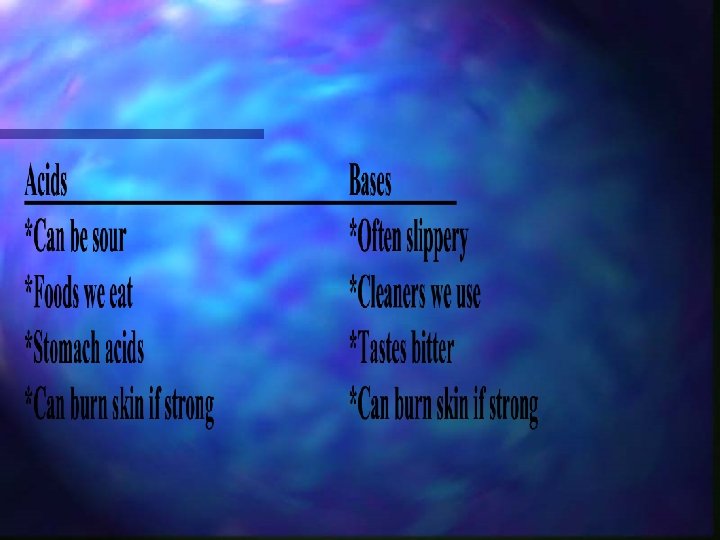
Acids n Bases Produces H+ ions in n Produces OH- ions in solution and have p. H values less than p. H values greater 7 than 7 + H 2 O H + OH Water hydrogen hydroxide *Pure water is NEUTRAL and has a p. H of 7.

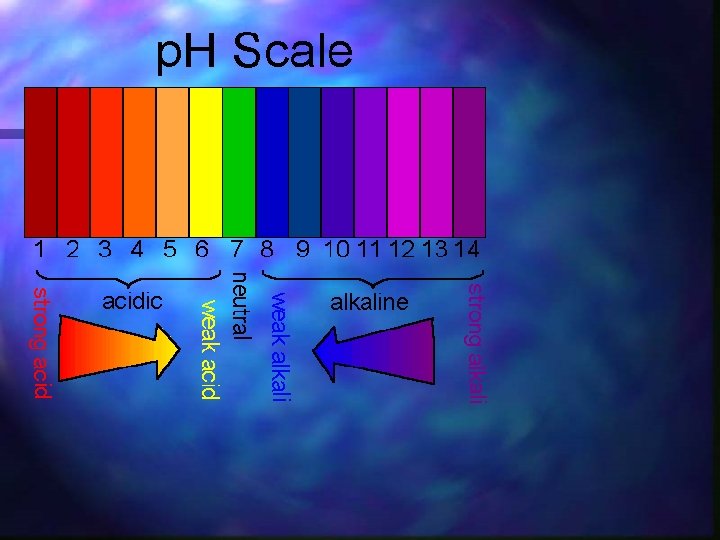
p. H Scale: Stronger 0 Acid Neutral 7 Stronger Base 14 NOTE: Every # is a factor of 10 X!


Why do we care in BIOLOGY? n n n Stomach is acidic (help break down food) Soil p. H is key to plant life (Hydrangeas change color based on p. H of soil) Acid Precipitation (affects both aquatic life and plants) Enzymes function at certain p. H Most biological processes take place in p. H close to NEUTRAL (6. 5 -7. 5), some slightly basic or acidic, in order to maintain homeostasis

What is a buffer? n Mixtures that can react with acids or bases to keep the p. H within a certain range n Example – buffers in blood to keep p. H about 7. 4


Let’s Watch!! n http: //www. youtube. com/watch? v=z. TLi JE-j 1 I&feature=Play. List&p=37 CBB 4 FE 24 FC 3 885&playnext=1&playnext_from=PL&in dex=42