Properties of Water Polarity Hbonds Water Density Water
Properties of Water • • • Polarity & H-bonds Water Density Water as a Solvent Heat (Sensible vs Latent) Surface Tension Reynolds Number (Inertia to Viscosity) Molecular Diffusion Flow Type (Laminar vs Turbulent) Light Attenuation
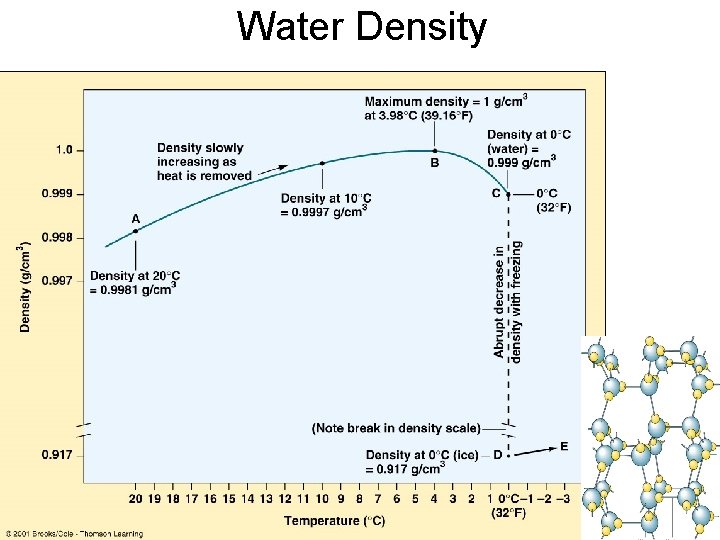
Water Density
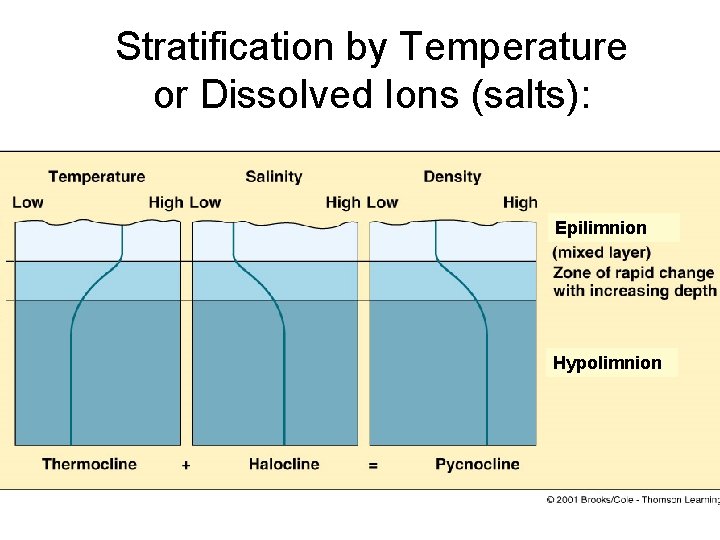
Stratification by Temperature or Dissolved Ions (salts): Epilimnion Hypolimnion
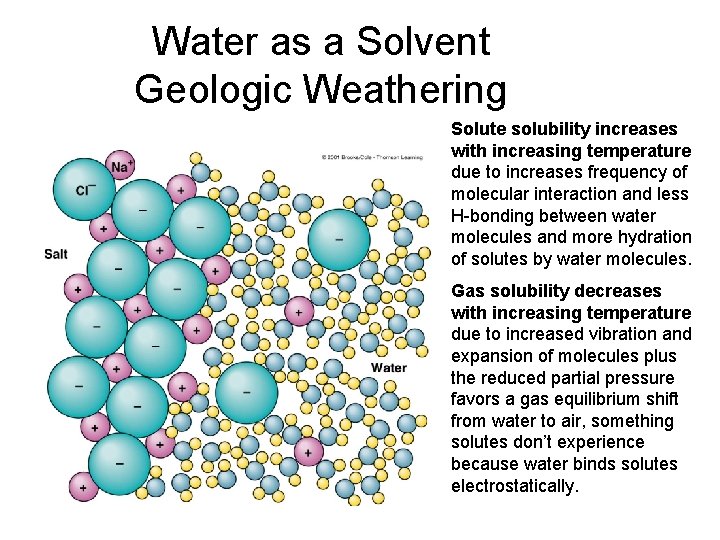
Water as a Solvent Geologic Weathering Solute solubility increases with increasing temperature due to increases frequency of molecular interaction and less H-bonding between water molecules and more hydration of solutes by water molecules. Gas solubility decreases with increasing temperature due to increased vibration and expansion of molecules plus the reduced partial pressure favors a gas equilibrium shift from water to air, something solutes don’t experience because water binds solutes electrostatically.

Surface Tension • At water-air or water-solid interfaces molecules of water are nearly completely H-bonded together, like a molecule thick layer of ice. • This is added tension force is utilized by many aquatic & semiaquatic arthropods Water strider (right) Fishing Spider (left) • It is also what explains waters ability to be drawn against gravity when in narrow spaces; capillary action.
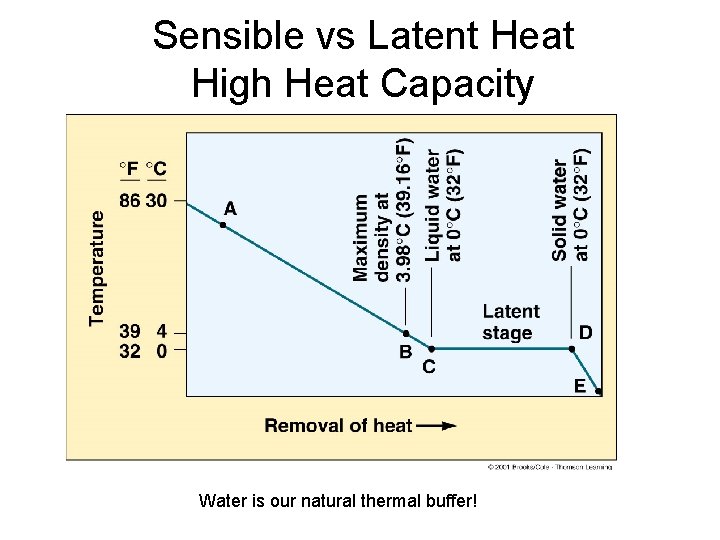
Sensible vs Latent Heat High Heat Capacity Water is our natural thermal buffer!
- Slides: 6