Properties of Water Notes Objectives Recognize the importance

Properties of Water Notes

Objectives • Recognize the importance of hydrogen bonding. • Explain why many compounds dissolve in water. • Compare acids & bases.

Vocabulary • • Hydrogen bond Solution Solvent Solute Acid Base p. H

Life depends on hydrogen bonds in water.
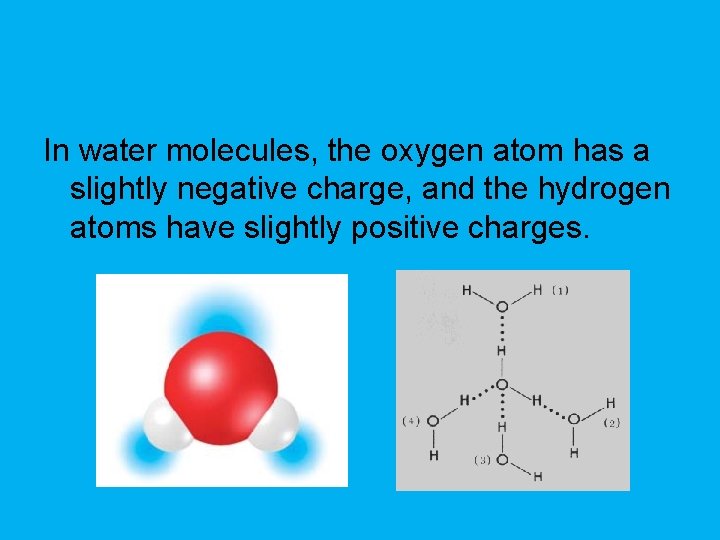
In water molecules, the oxygen atom has a slightly negative charge, and the hydrogen atoms have slightly positive charges.

Hydrogen bond • Attraction between a slightly positive hydrogen atom and a slightly negative atom.

Water’s surface tension comes from hydrogen bonds that cause water molecules to stick together.

Many compounds dissolve in water.

Solution • Mixture of substances in which the components are evenly distributed throughout.

Solvent • Substance in a solution that is present in the greater amount and that dissolves another substance.

Solute • Substance in a solution that dissolves and is present at a lower concentration than the solvent.

Some compounds form acids or bases.
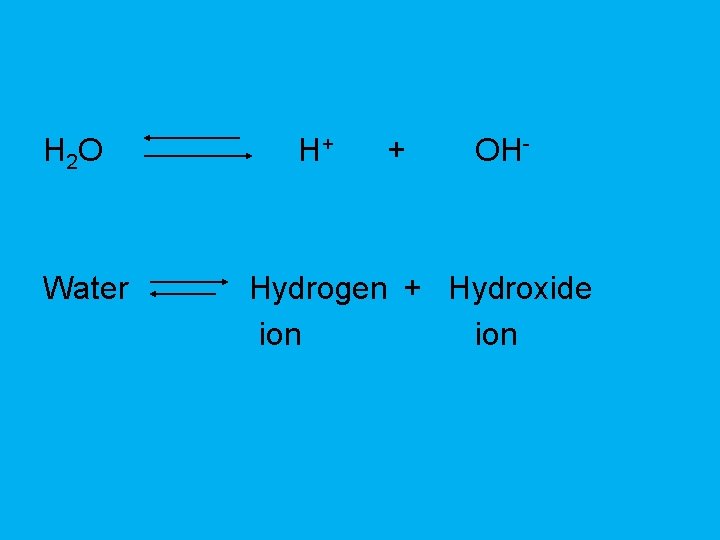
H 2 O Water H+ + OH- Hydrogen + Hydroxide ion

Acid • Any compound that releases H+ ions in solution.

Base • Compound that removes H+ ions from a solution.

p. H • Measurement of acidity; related to free hydrogen ion concentration in solution.
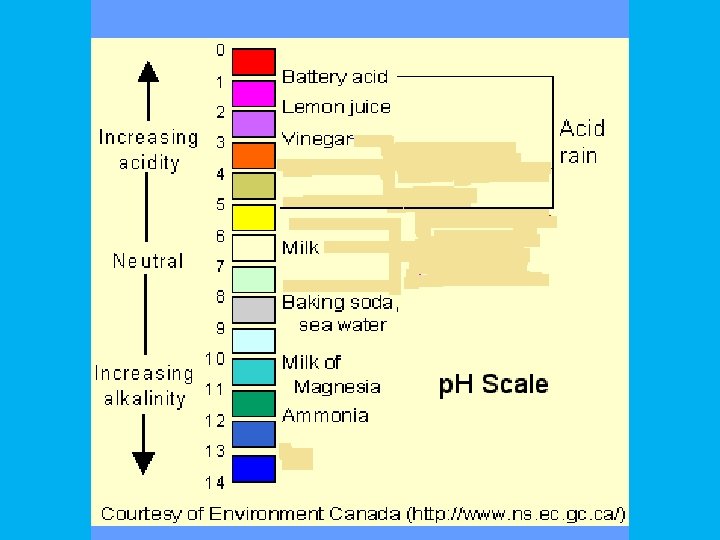

Acidic solutions contain higher concentrations of H+ ions than pure water and have p. H values below 7.

Basic, or alkaline, solutions contain lower concentrations of H+ ions than pure water and have p. H values above 7.
- Slides: 19