Properties of Solutions Solution q A solution is


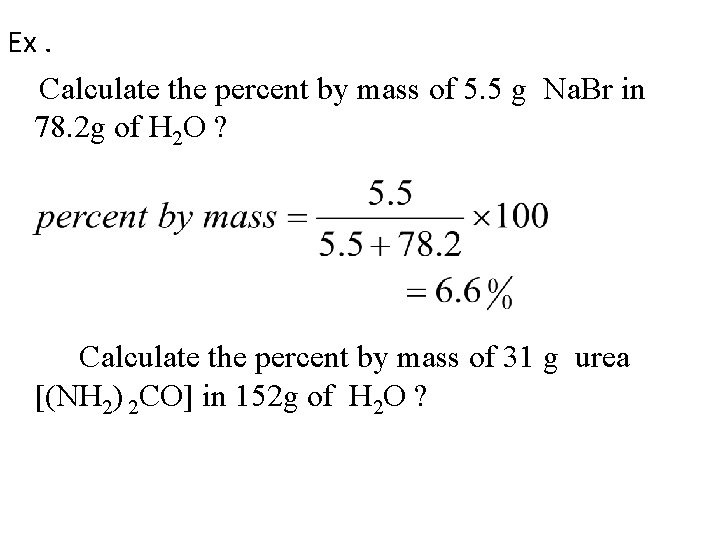

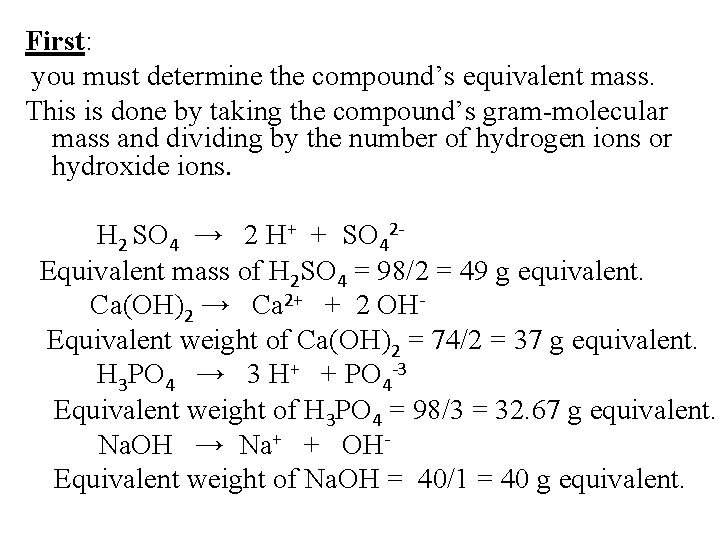




- Slides: 29

Properties of Solutions

Solution q A solution : is a homogeneous mixture of two or more substances. q The solute : is the substance present in a small amount. q The solvent : is the substance present in a large amount. q. In aqueous solutions, the solute is a liquid or solid and the solvent is water.

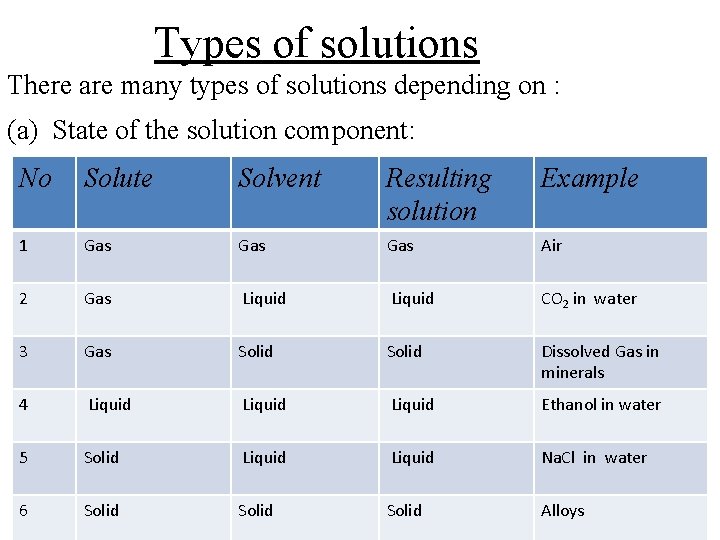
Types of solutions There are many types of solutions depending on : (a) State of the solution component: No Solute Solvent Resulting solution Example 1 Gas Gas Air 2 Gas Liquid CO 2 in water 3 Gas Solid Dissolved Gas in minerals 4 Liquid Ethanol in water 5 Solid Liquid Na. Cl in water 6 Solid Alloys

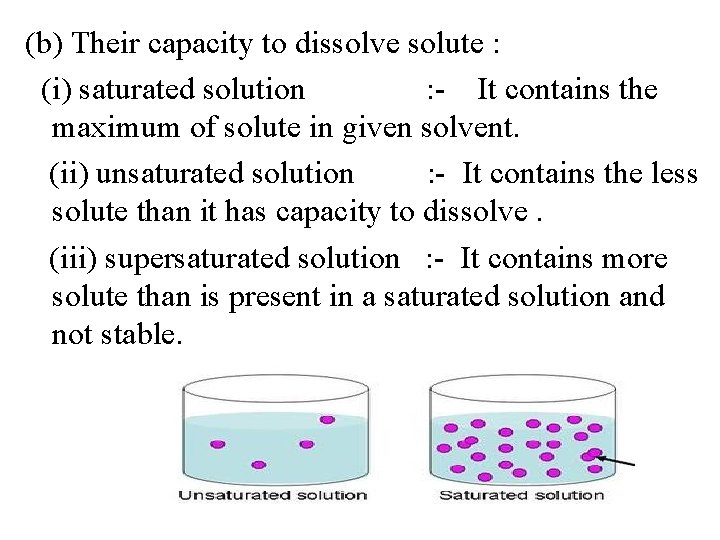
(b) Their capacity to dissolve solute : (i) saturated solution : - It contains the maximum of solute in given solvent. (ii) unsaturated solution : - It contains the less solute than it has capacity to dissolve. (iii) supersaturated solution : - It contains more solute than is present in a saturated solution and not stable.

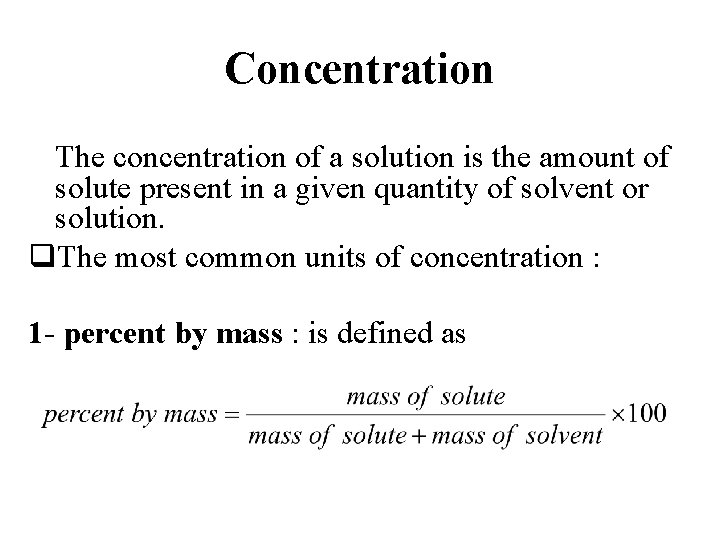
Concentration The concentration of a solution is the amount of solute present in a given quantity of solvent or solution. q. The most common units of concentration : 1 - percent by mass : is defined as
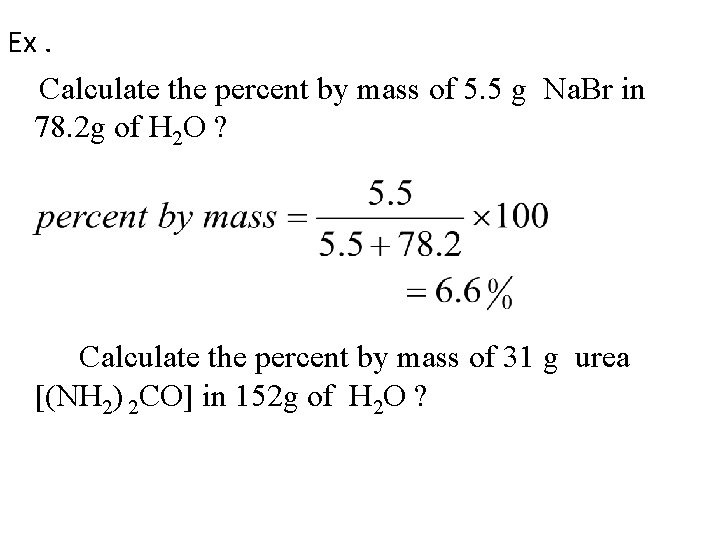
Ex. Calculate the percent by mass of 5. 5 g Na. Br in 78. 2 g of H 2 O ? Calculate the percent by mass of 31 g urea [(NH 2) 2 CO] in 152 g of H 2 O ?

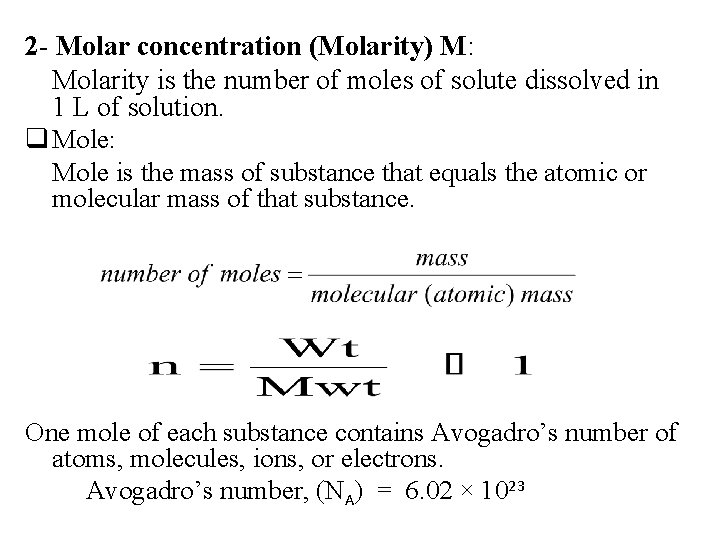
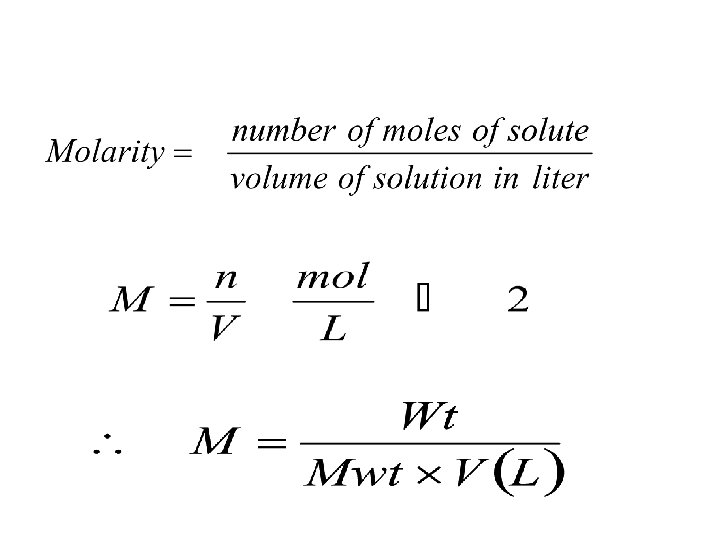
2 - Molar concentration (Molarity) M: Molarity is the number of moles of solute dissolved in 1 L of solution. q Mole: Mole is the mass of substance that equals the atomic or molecular mass of that substance. One mole of each substance contains Avogadro’s number of atoms, molecules, ions, or electrons. Avogadro’s number, (NA) = 6. 02 × 1023


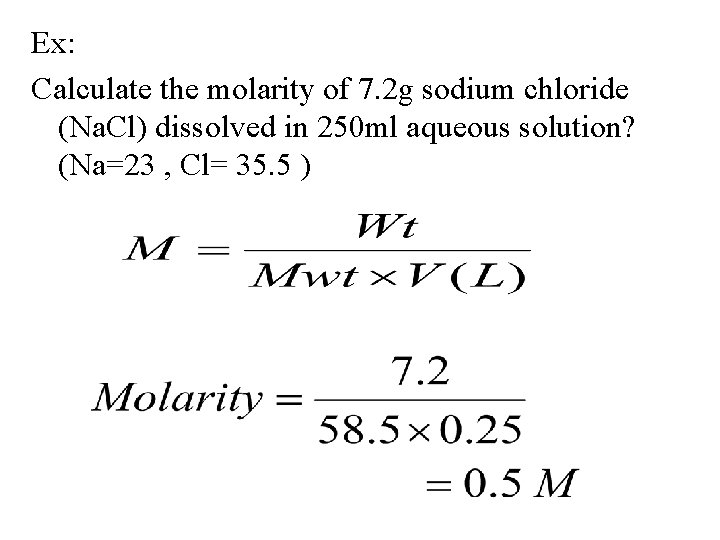
Ex: Calculate the molarity of 7. 2 g sodium chloride (Na. Cl) dissolved in 250 ml aqueous solution? (Na=23 , Cl= 35. 5 )

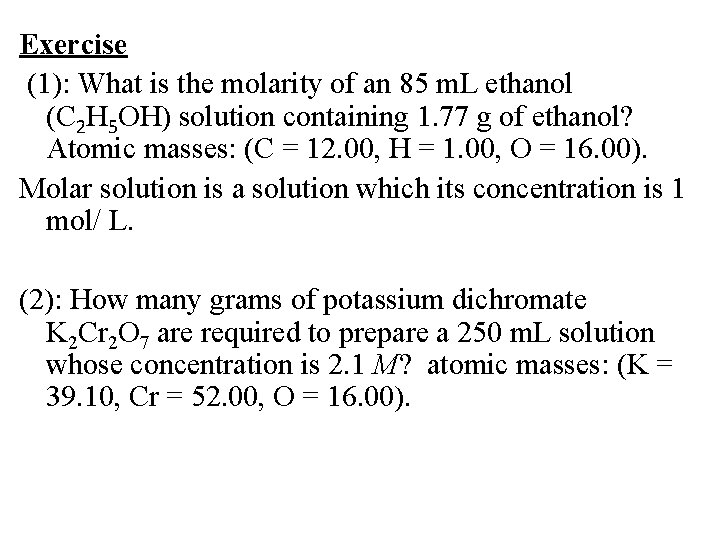
Exercise (1): What is the molarity of an 85 m. L ethanol (C 2 H 5 OH) solution containing 1. 77 g of ethanol? Atomic masses: (C = 12. 00, H = 1. 00, O = 16. 00). Molar solution is a solution which its concentration is 1 mol/ L. (2): How many grams of potassium dichromate K 2 Cr 2 O 7 are required to prepare a 250 m. L solution whose concentration is 2. 1 M? atomic masses: (K = 39. 10, Cr = 52. 00, O = 16. 00).

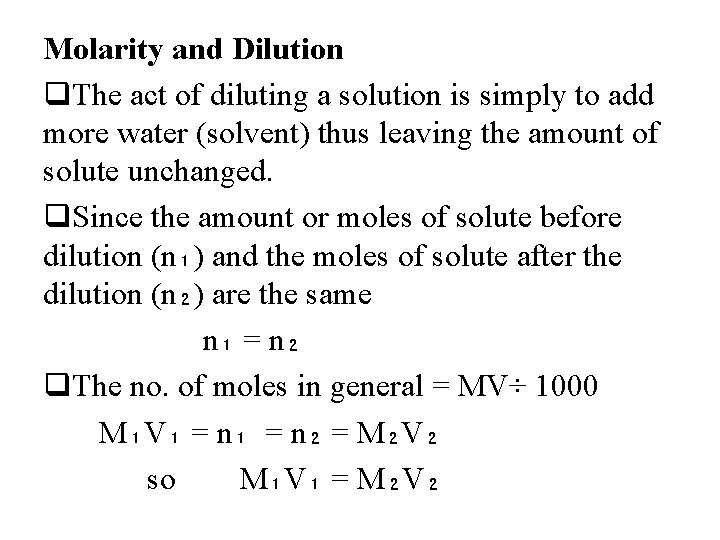
Molarity and Dilution q. The act of diluting a solution is simply to add more water (solvent) thus leaving the amount of solute unchanged. q. Since the amount or moles of solute before dilution (n₁) and the moles of solute after the dilution (n₂) are the same n₁ = n₂ q. The no. of moles in general = MV÷ 1000 M₁V₁ = n₂ = M₂V₂ so M₁V₁ = M₂V₂

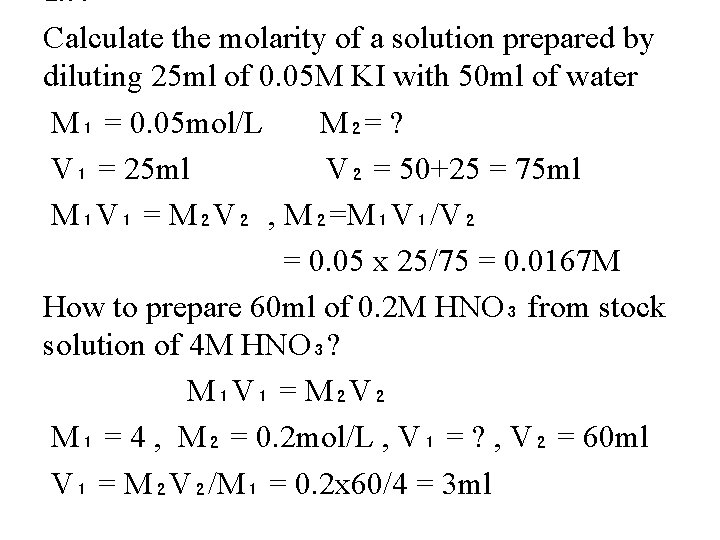
Ex : Calculate the molarity of a solution prepared by diluting 25 ml of 0. 05 M KI with 50 ml of water M₁ = 0. 05 mol/L M₂= ? V₁ = 25 ml V₂ = 50+25 = 75 ml M₁V₁ = M₂V₂ , M₂=M₁V₁/V₂ = 0. 05 x 25/75 = 0. 0167 M How to prepare 60 ml of 0. 2 M HNO₃ from stock solution of 4 M HNO₃? M₁V₁ = M₂V₂ M₁ = 4 , M₂ = 0. 2 mol/L , V₁ = ? , V₂ = 60 ml V₁ = M₂V₂/M₁ = 0. 2 x 60/4 = 3 ml

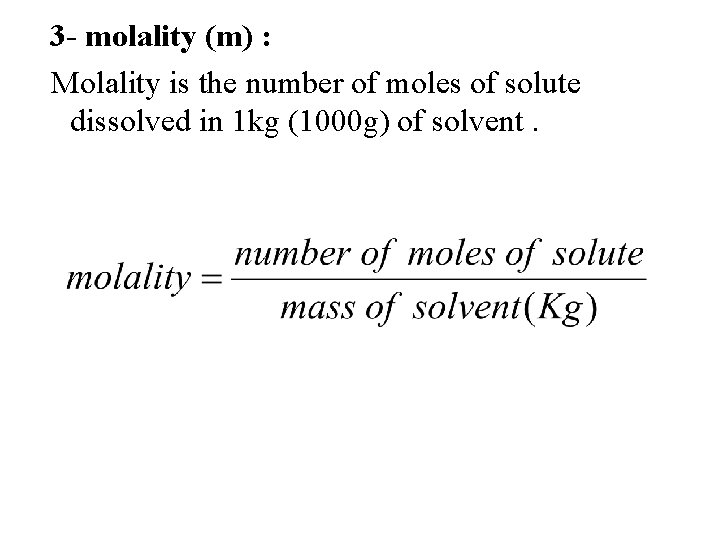
3 - molality (m) : Molality is the number of moles of solute dissolved in 1 kg (1000 g) of solvent.

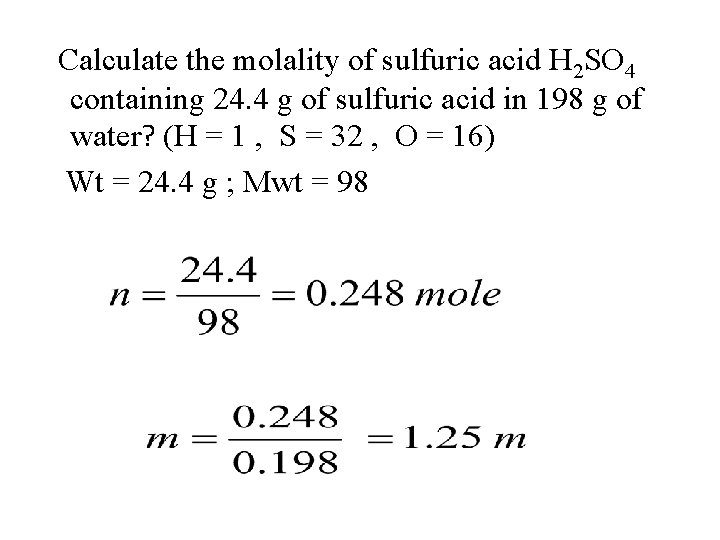
Calculate the molality of sulfuric acid H 2 SO 4 containing 24. 4 g of sulfuric acid in 198 g of water? (H = 1 , S = 32 , O = 16) Wt = 24. 4 g ; Mwt = 98

Exercise: What is the molality of a solution containing 7. 78 g of urea [(NH 2)2 CO] in 203 g of water. Molal solution is the solution which its concentration is 1 mol/ kg.

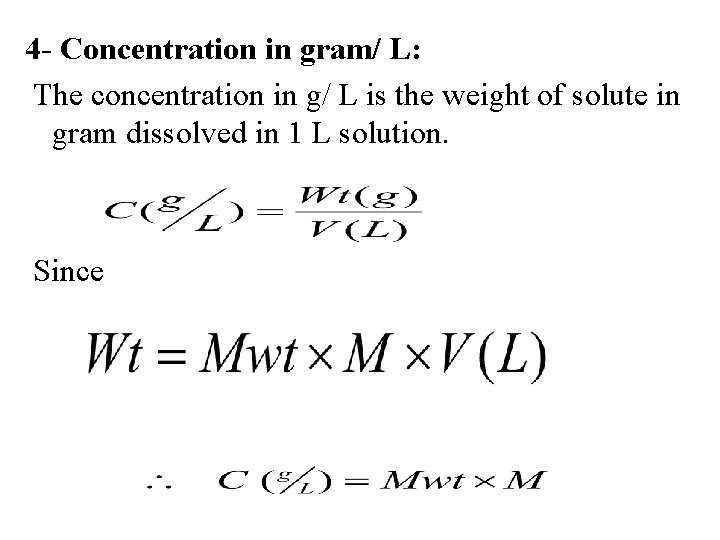
4 - Concentration in gram/ L: The concentration in g/ L is the weight of solute in gram dissolved in 1 L solution. Since

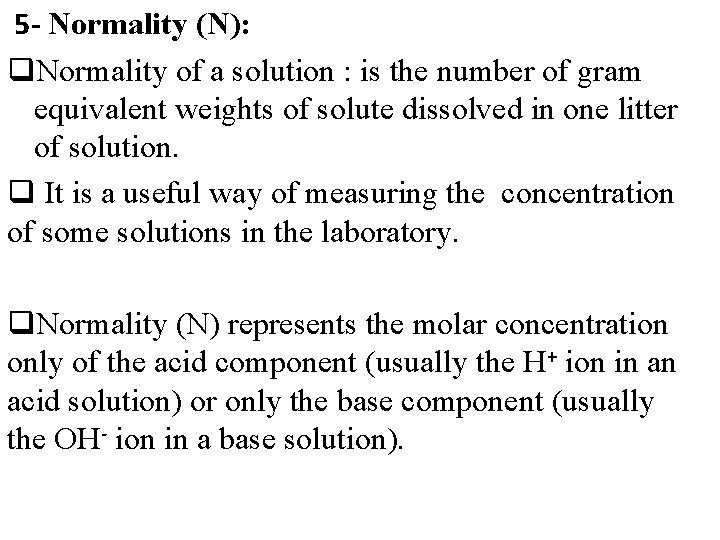
5 - Normality (N): q. Normality of a solution : is the number of gram equivalent weights of solute dissolved in one litter of solution. q It is a useful way of measuring the concentration of some solutions in the laboratory. q. Normality (N) represents the molar concentration only of the acid component (usually the H+ ion in an acid solution) or only the base component (usually the OH- ion in a base solution).
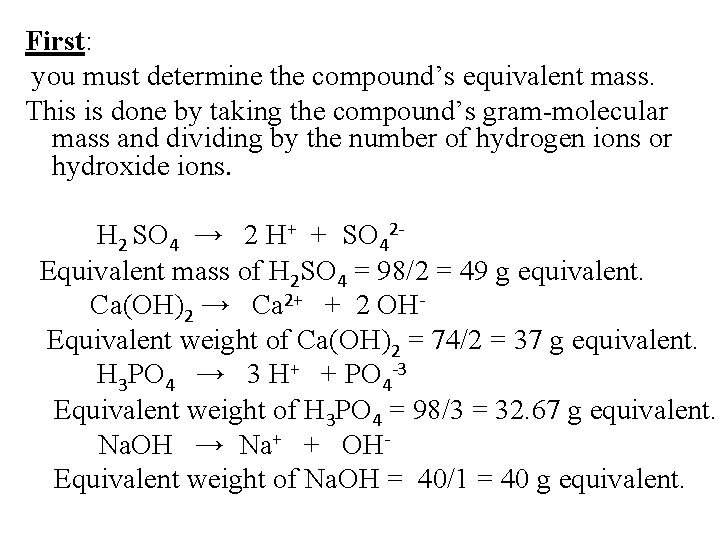
First: you must determine the compound’s equivalent mass. This is done by taking the compound’s gram-molecular mass and dividing by the number of hydrogen ions or hydroxide ions. H 2 SO 4 → 2 H+ + SO 42 Equivalent mass of H 2 SO 4 = 98/2 = 49 g equivalent. Ca(OH)2 → Ca 2+ + 2 OHEquivalent weight of Ca(OH)2 = 74/2 = 37 g equivalent. H 3 PO 4 → 3 H+ + PO 4 -3 Equivalent weight of H 3 PO 4 = 98/3 = 32. 67 g equivalent. Na. OH → Na+ + OHEquivalent weight of Na. OH = 40/1 = 40 g equivalent.

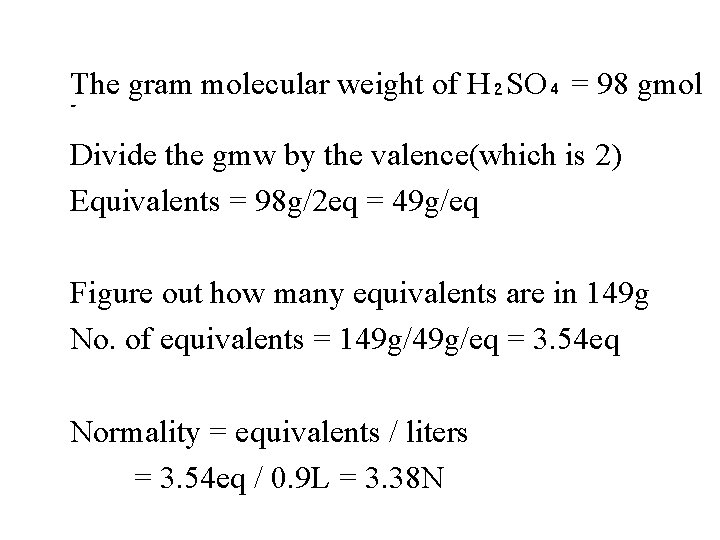
second : you can then calculate No. of equivalents = grams/ equivalent mass. Third : Normality = equivalents / liters. Ex: what is the normality of a solution containing 149 g of H₂SO₄ in 900 ml ?

The gram molecular weight of H₂SO₄ = 98 gmol - Divide the gmw by the valence(which is 2) Equivalents = 98 g/2 eq = 49 g/eq Figure out how many equivalents are in 149 g No. of equivalents = 149 g/eq = 3. 54 eq Normality = equivalents / liters = 3. 54 eq / 0. 9 L = 3. 38 N

q. If you know the Molarity of an acid or base solution, you can easily convert it to Normality by multiplying Molarity by the number of hydrogen (or hydroxide) ions in the acid (or base). N = (M)(number of hydrogen or hydroxide For. ions) q example, a 2 M H 2 SO 4 solution will have a Normality of 4 N (2 M x 2 hydrogen ions). 2 M H 3 PO 4, solution will have a Normality of 6 N

Problems 1 -What is the concentration of 35 ml of 0. 055 moles of KCl ? 2 -How many grams of KCl is needed to prepare 50 ml of a 0. 1 M solution ? 3 -How many milliliters of water must be added to 30 ml of 9 MKCl to make a solution that is 0. 5 M?

Standard solution: Standard solution is a solution of accurately known concentration. Titration: In a titration, a solution of standard is added gradually to another solution of unknown concentration until the chemical reaction between the two solutions is complete.

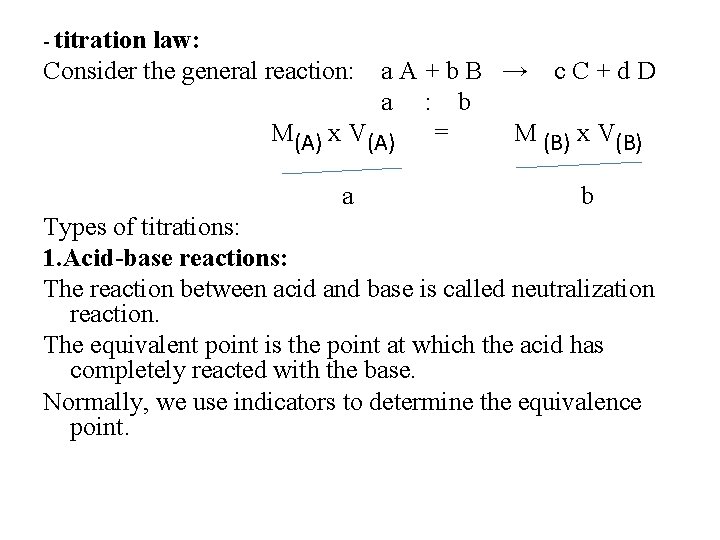
- titration law: Consider the general reaction: a. A+b. B → c. C+d. D a : b M(A) x V(A) = M (B) x V(B) a b Types of titrations: 1. Acid-base reactions: The reaction between acid and base is called neutralization reaction. The equivalent point is the point at which the acid has completely reacted with the base. Normally, we use indicators to determine the equivalence point.

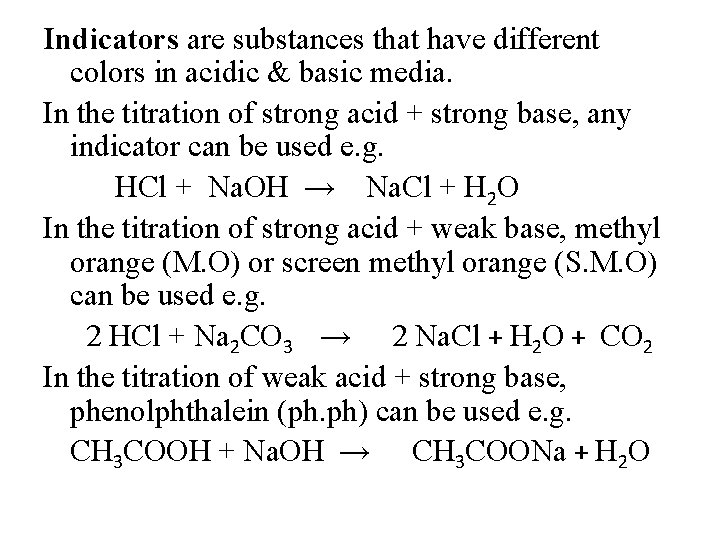
Indicators are substances that have different colors in acidic & basic media. In the titration of strong acid + strong base, any indicator can be used e. g. HCl + Na. OH → Na. Cl + H 2 O In the titration of strong acid + weak base, methyl orange (M. O) or screen methyl orange (S. M. O) can be used e. g. 2 HCl + Na 2 CO 3 → 2 Na. Cl + H 2 O + CO 2 In the titration of weak acid + strong base, phenolphthalein (ph. ph) can be used e. g. CH 3 COOH + Na. OH → CH 3 COONa + H 2 O

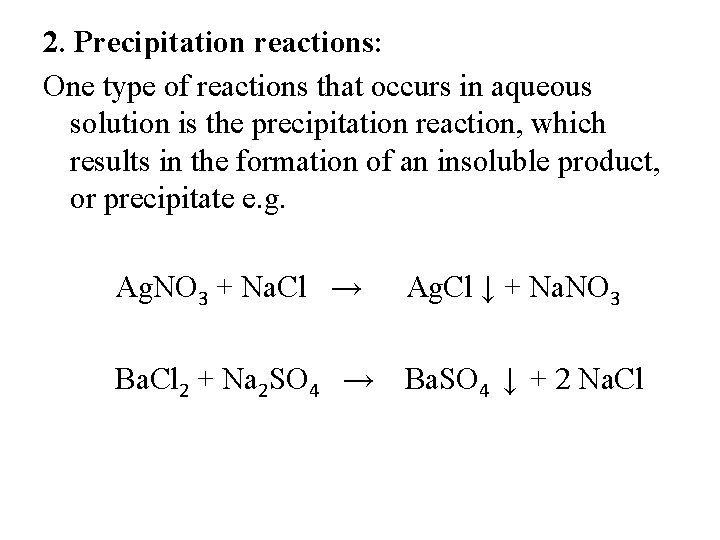
2. Precipitation reactions: One type of reactions that occurs in aqueous solution is the precipitation reaction, which results in the formation of an insoluble product, or precipitate e. g. Ag. NO 3 + Na. Cl → Ag. Cl ↓ + Na. NO 3 Ba. Cl 2 + Na 2 SO 4 → Ba. SO 4 ↓ + 2 Na. Cl

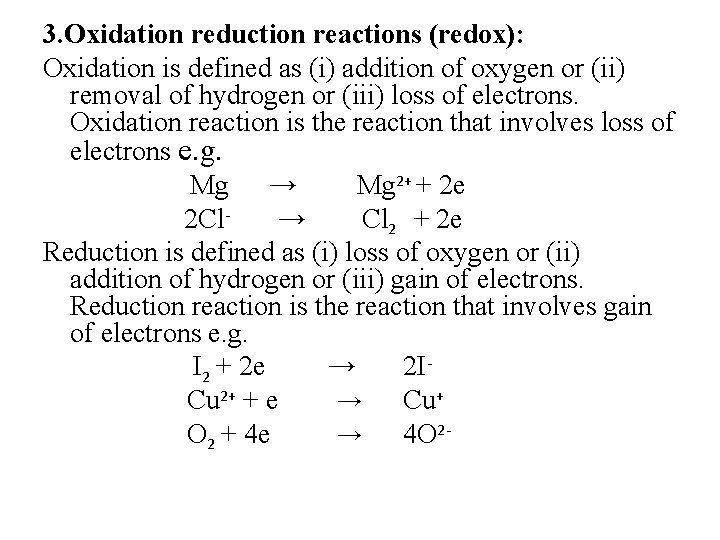
3. Oxidation reduction reactions (redox): Oxidation is defined as (i) addition of oxygen or (ii) removal of hydrogen or (iii) loss of electrons. Oxidation reaction is the reaction that involves loss of electrons e. g. Mg → Mg 2+ + 2 e 2 Cl→ Cl 2 + 2 e Reduction is defined as (i) loss of oxygen or (ii) addition of hydrogen or (iii) gain of electrons. Reduction reaction is the reaction that involves gain of electrons e. g. I 2 + 2 e → 2 ICu 2+ + e → Cu+ O 2 + 4 e → 4 O 2 -

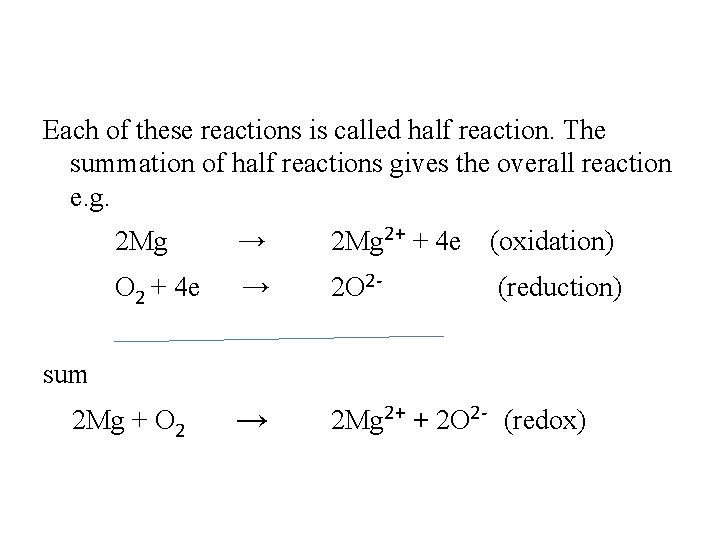
Each of these reactions is called half reaction. The summation of half reactions gives the overall reaction e. g. 2 Mg → 2 Mg 2+ + 4 e (oxidation) O 2 + 4 e → 2 O 2 - (reduction) → 2 Mg 2+ + 2 O 2 - (redox) sum 2 Mg + O 2

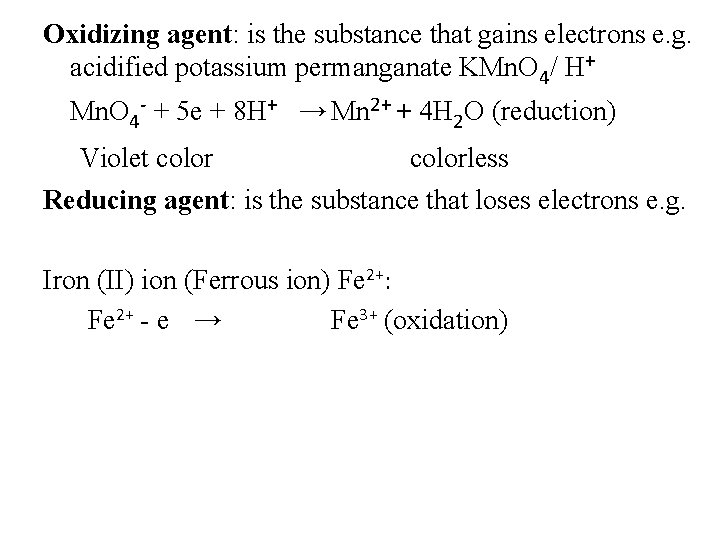
Oxidizing agent: is the substance that gains electrons e. g. acidified potassium permanganate KMn. O 4/ H+ Mn. O 4 - + 5 e + 8 H+ → Mn 2+ + 4 H 2 O (reduction) Violet colorless Reducing agent: is the substance that loses electrons e. g. Iron (ІІ) ion (Ferrous ion) Fe 2+: Fe 2+ - e → Fe 3+ (oxidation)