PROPERTIES OF SOLIDS SCH 4 U 1 Intra








- Slides: 40

PROPERTIES OF SOLIDS SCH 4 U 1

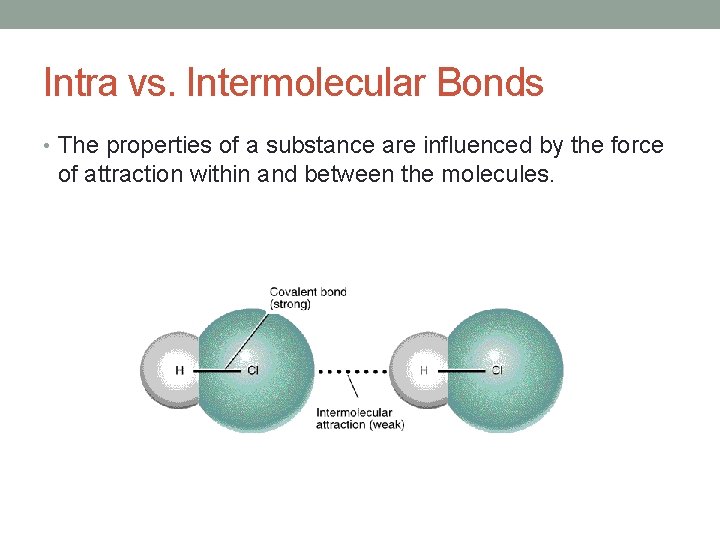
Intra vs. Intermolecular Bonds • The properties of a substance are influenced by the force of attraction within and between the molecules.

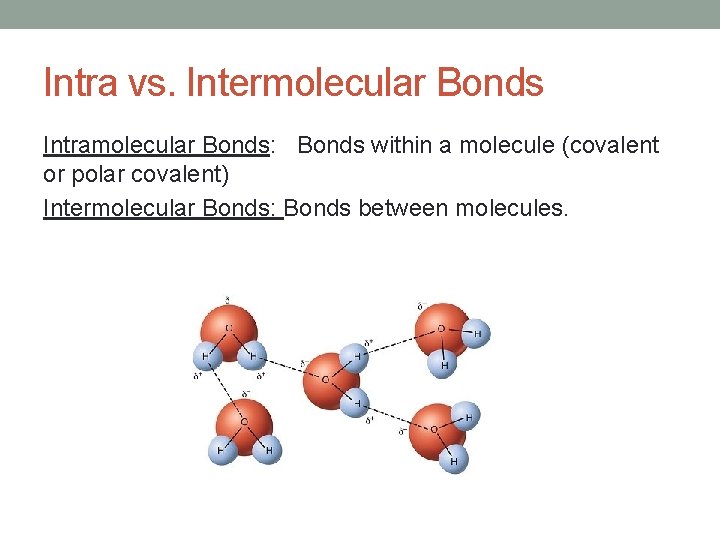
Intra vs. Intermolecular Bonds Intramolecular Bonds: Bonds within a molecule (covalent or polar covalent) Intermolecular Bonds: Bonds between molecules.

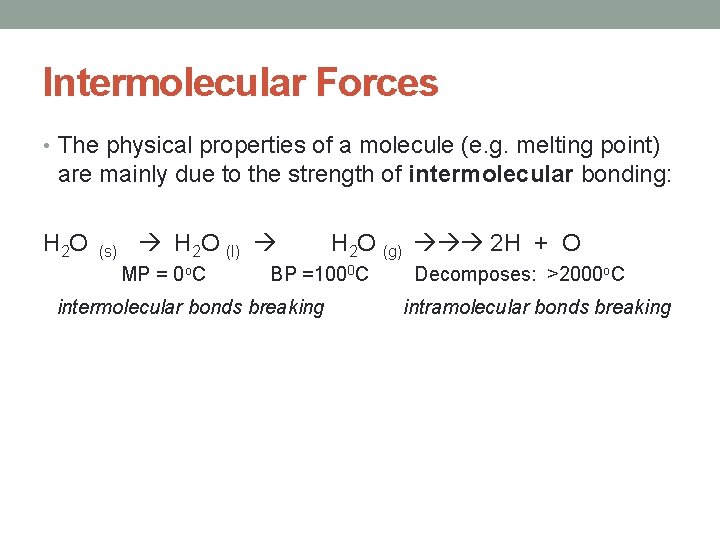
Intermolecular Forces • The physical properties of a molecule (e. g. melting point) are mainly due to the strength of intermolecular bonding: H 2 O (s) H 2 O (l) MP = 0 o. C H 2 O (g) 2 H + O BP =1000 C intermolecular bonds breaking Decomposes: >2000 o. C intramolecular bonds breaking

1) Atomic Solids • Noble gases form liquids and solids at very low temperature due to the very weak bonds between the atoms. • Since the attraction is so weak, they are weakened and broken at very low temperature. • E. g. argon (Ar): mp = -189 o. C; bp = -186 o. C

van der Waals (London) Forces • Since they do form solids, a very weak attraction must exist between the atoms. • These are explained by weak attractions between molecules called van der Waals forces. • London forces are the weakest type of van der Waals attraction.

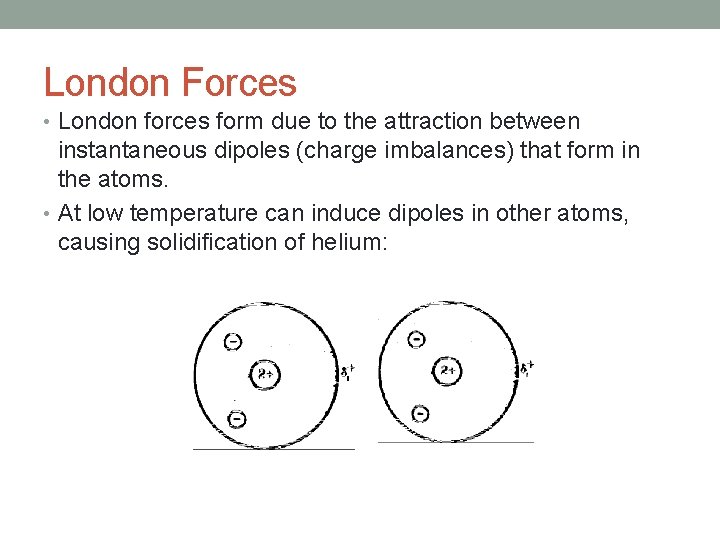
London Forces • London forces form due to the attraction between instantaneous dipoles (charge imbalances) that form in the atoms. • At low temperature can induce dipoles in other atoms, causing solidification of helium:

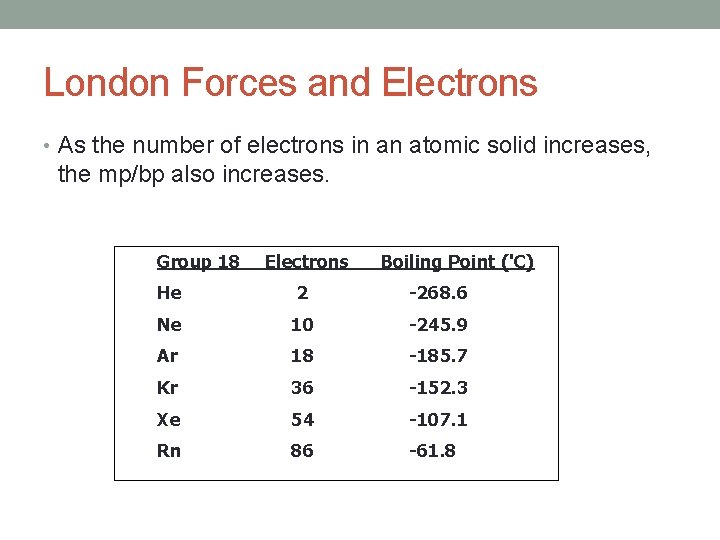
London Forces and Electrons • As the number of electrons in an atomic solid increases, the mp/bp also increases. Group 18 Electrons Boiling Point ('C) He 2 -268. 6 Ne 10 -245. 9 Ar 18 -185. 7 Kr 36 -152. 3 Xe 54 -107. 1 Rn 86 -61. 8

Summary: Properties of Atomic Solids • very low melting points/ boiling point • do not conduct electricity • mp/bp increase down the group (increasing London forces) • Liquid helium is a very strange substance.

Molecular Solids • Substances with covalent bonds or polar covalent bonds (N 2, CH 4, H 2 O, C 6 H 12 O 6 etc. ). • Can exist in all states at room temperature. Check out liquid nitrogen!

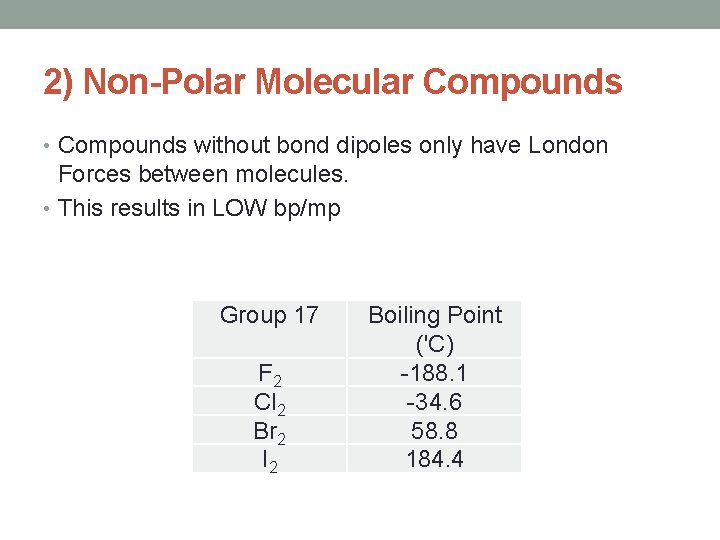
2) Non-Polar Molecular Compounds • Compounds without bond dipoles only have London Forces between molecules. • This results in LOW bp/mp Group 17 F 2 Cl 2 Br 2 I 2 Boiling Point ('C) -188. 1 -34. 6 58. 8 184. 4

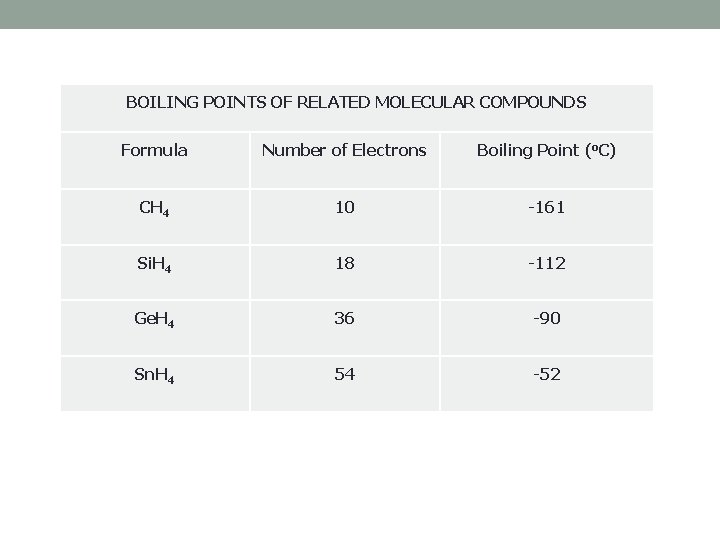
BOILING POINTS OF RELATED MOLECULAR COMPOUNDS Formula Number of Electrons Boiling Point (o. C) CH 4 10 -161 Si. H 4 18 -112 Ge. H 4 36 -90 Sn. H 4 54 -52

Comparing Larger Compounds • When comparing non-polar compounds, the forces of attraction are greater between molecules with the greatest number of atoms. • There are more locations for London (van der Waals) forces to occur between adjacent molecules.

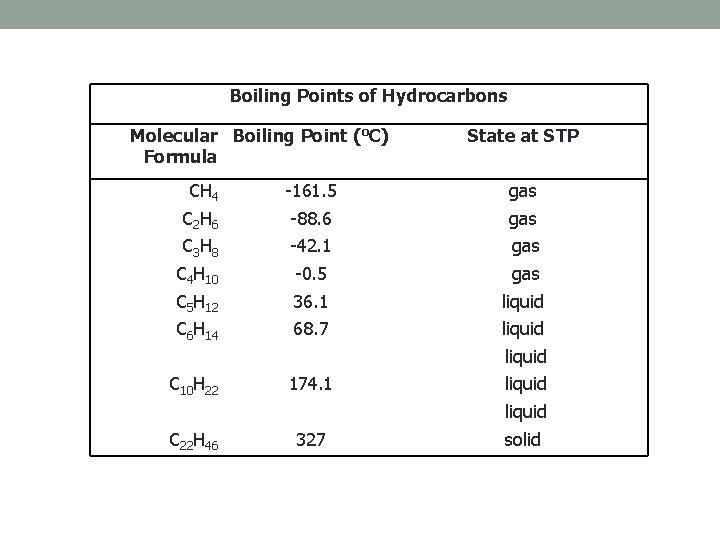
Boiling Points of Hydrocarbons Molecular Boiling Point (o. C) Formula State at STP CH 4 -161. 5 gas C 2 H 6 -88. 6 gas C 3 H 8 -42. 1 gas C 4 H 10 -0. 5 gas C 5 H 12 36. 1 liquid C 6 H 14 68. 7 liquid 174. 1 liquid 327 solid C 10 H 22 C 22 H 46

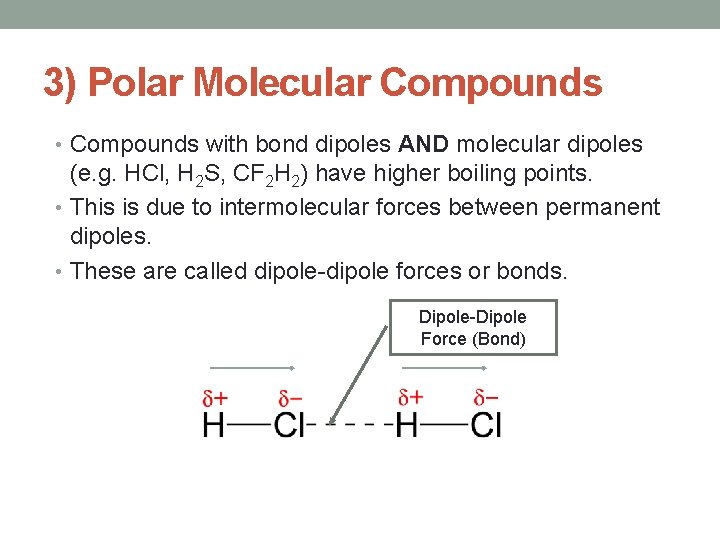
3) Polar Molecular Compounds • Compounds with bond dipoles AND molecular dipoles (e. g. HCl, H 2 S, CF 2 H 2) have higher boiling points. • This is due to intermolecular forces between permanent dipoles. • These are called dipole-dipole forces or bonds. Dipole-Dipole Force (Bond)

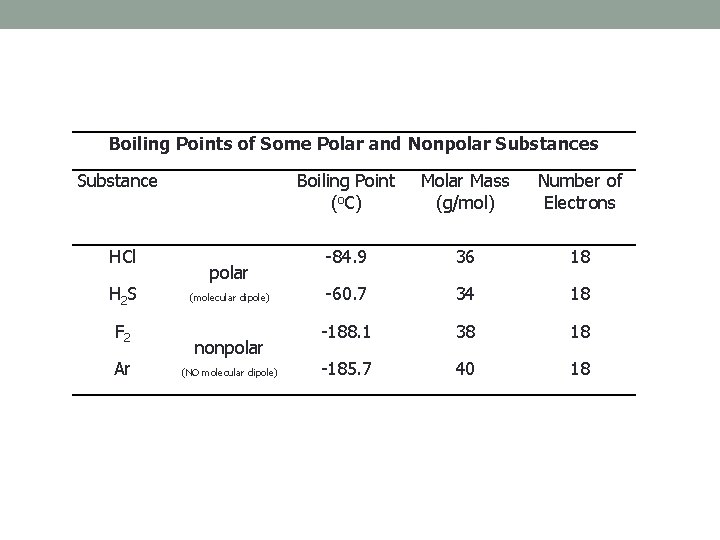
Boiling Points of Some Polar and Nonpolar Substances Substance HCl H 2 S F 2 Ar polar (molecular dipole) nonpolar (NO molecular dipole) Boiling Point (o. C) Molar Mass (g/mol) Number of Electrons -84. 9 36 18 -60. 7 34 18 -188. 1 38 18 -185. 7 40 18

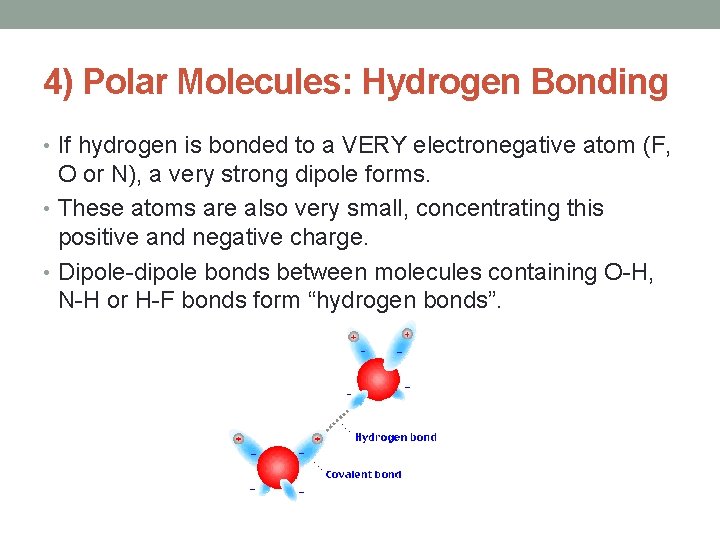
4) Polar Molecules: Hydrogen Bonding • If hydrogen is bonded to a VERY electronegative atom (F, O or N), a very strong dipole forms. • These atoms are also very small, concentrating this positive and negative charge. • Dipole-dipole bonds between molecules containing O-H, N-H or H-F bonds form “hydrogen bonds”.

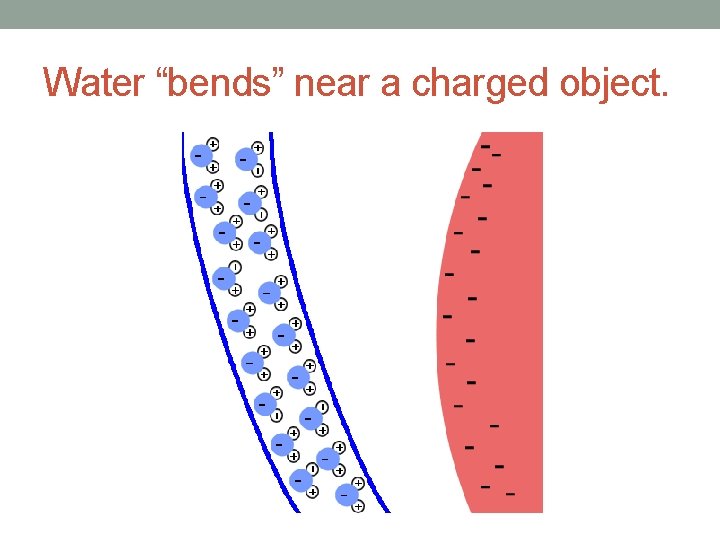
Water “bends” near a charged object.

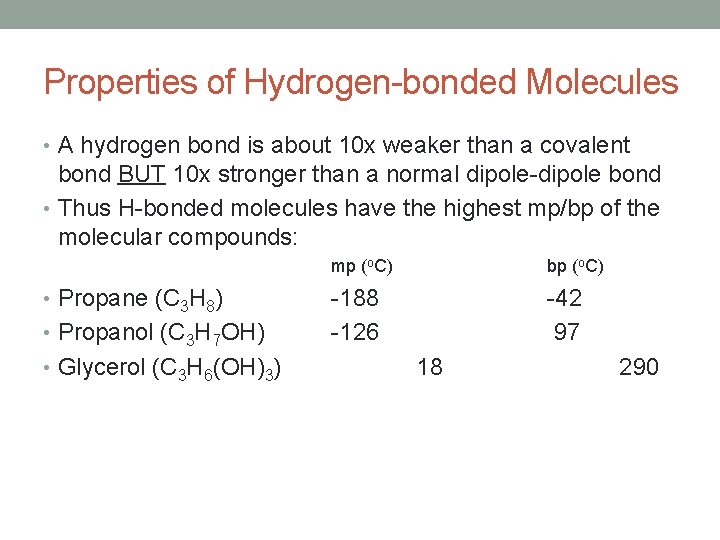
Properties of Hydrogen-bonded Molecules • A hydrogen bond is about 10 x weaker than a covalent bond BUT 10 x stronger than a normal dipole-dipole bond • Thus H-bonded molecules have the highest mp/bp of the molecular compounds: • Propane (C 3 H 8) • Propanol (C 3 H 7 OH) • Glycerol (C 3 H 6(OH)3) mp (o. C) bp (o. C) -188 -126 -42 97 18 290

General Properties of Molecular Compounds • Molecular compounds do not conduct electricity sine their electrons cannot move between molecules. • They have relatively low bp/mp due to the existence of weaker intermolecular forces • As the strength of these intermolecular forces increase, so does the mp and bp.

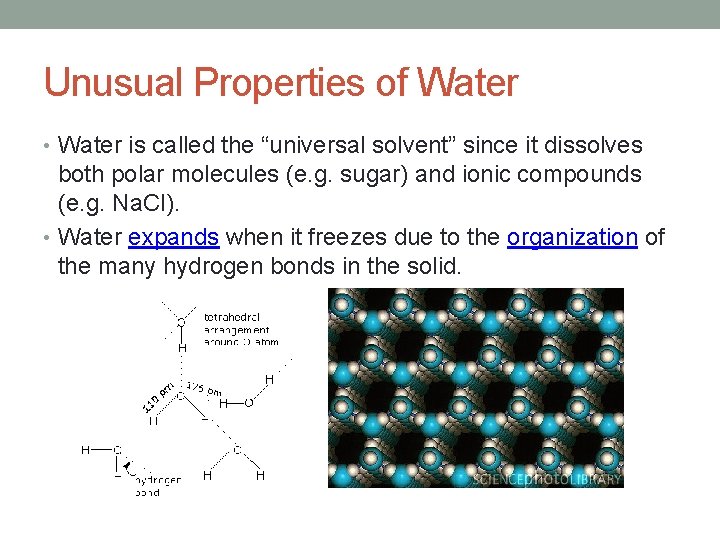
Unusual Properties of Water • Water is called the “universal solvent” since it dissolves both polar molecules (e. g. sugar) and ionic compounds (e. g. Na. Cl). • Water expands when it freezes due to the organization of the many hydrogen bonds in the solid.

5) Metallic Solids General Properties: • Few valence electrons • Low ionization energies • Malleable, ductile and shiny • Moderate mp/bp • Good conductors of heat and electricity in the solid and liquid states.

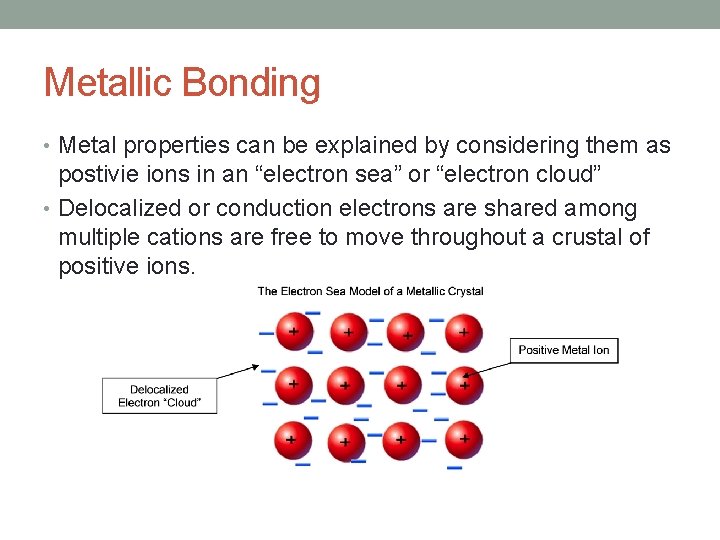
Metallic Bonding • Metal properties can be explained by considering them as postivie ions in an “electron sea” or “electron cloud” • Delocalized or conduction electrons are shared among multiple cations are free to move throughout a crustal of positive ions.

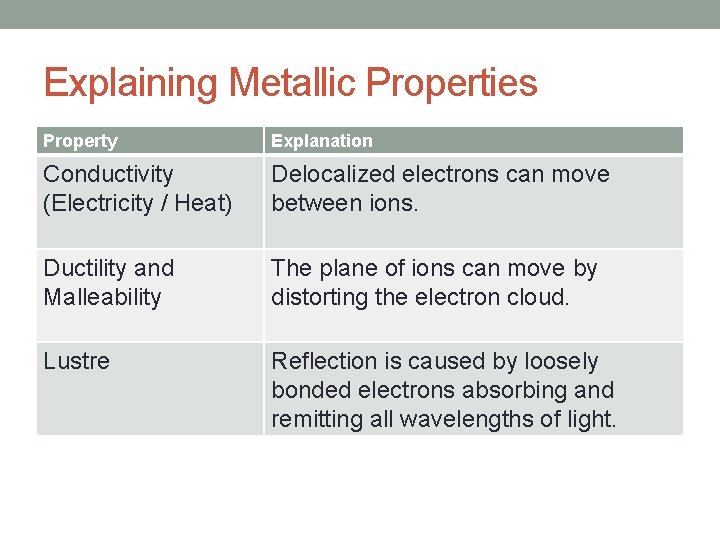
Explaining Metallic Properties Property Explanation Conductivity (Electricity / Heat) Delocalized electrons can move between ions. Ductility and Malleability The plane of ions can move by distorting the electron cloud. Lustre Reflection is caused by loosely bonded electrons absorbing and remitting all wavelengths of light.

• e. g. 1 Lithium is far more malleable than aluminum. Propose an explanation for this observation using the model of metallic bonding. Metallic bonding occurs since the loosely held (delocalized) electrons are mutually shared by a crystal of positive ions. Since Li has only 1 delocalized valence electron compared with aluminum which has 3 and aluminum has a greater nuclear charge, we can deduce that the additional protons & electrons strengthen the metallic bonding and make it more difficult to displace the network of atoms in the crystal.

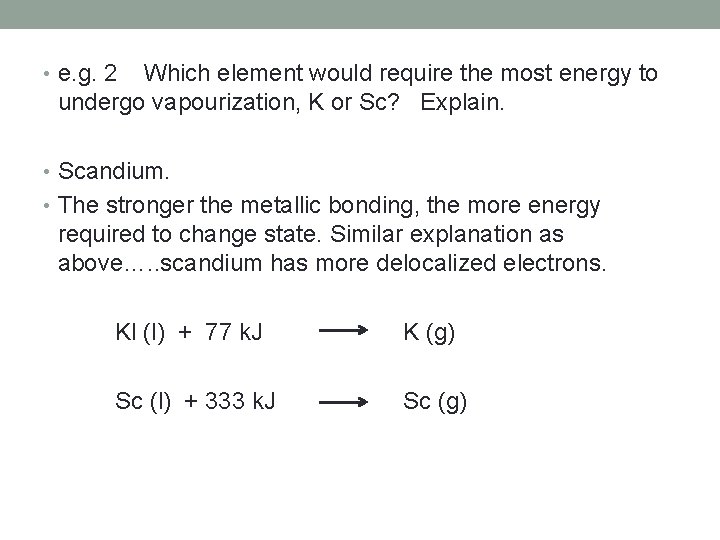
• e. g. 2 Which element would require the most energy to undergo vapourization, K or Sc? Explain. • Scandium. • The stronger the metallic bonding, the more energy required to change state. Similar explanation as above…. . scandium has more delocalized electrons. Kl (l) + 77 k. J K (g) Sc (l) + 333 k. J Sc (g)

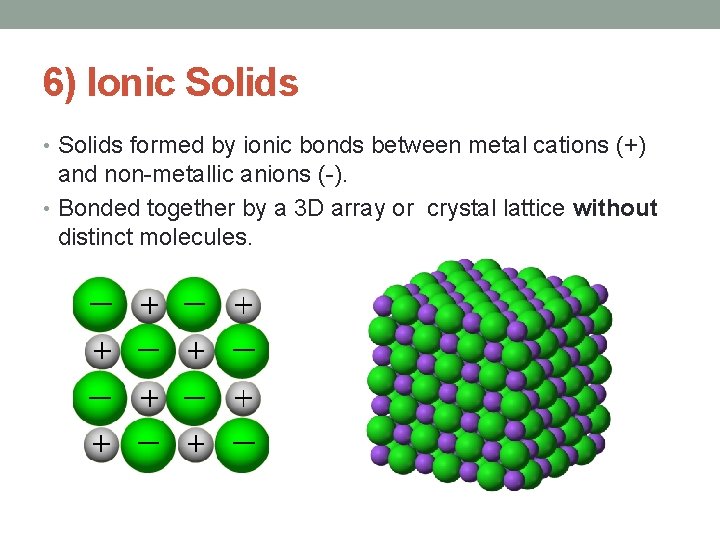
6) Ionic Solids • Solids formed by ionic bonds between metal cations (+) and non-metallic anions (-). • Bonded together by a 3 D array or crystal lattice without distinct molecules.

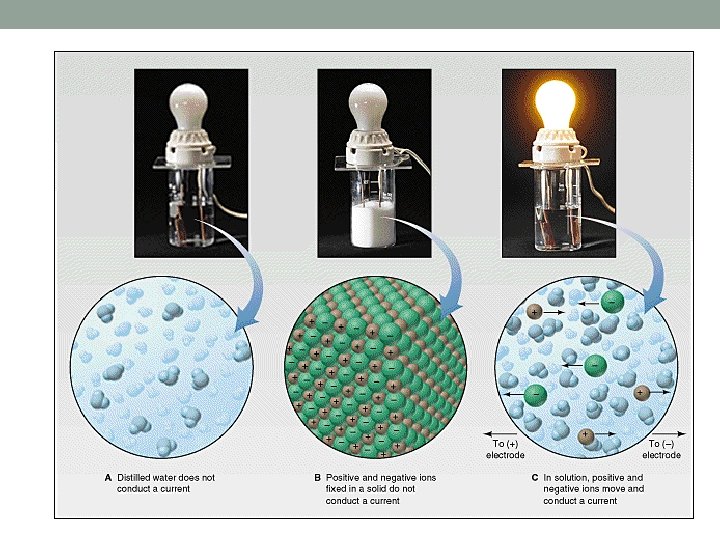
Properties of Ionic Solids • High melting points and boiling points (many ionic bonds that must be broken to change states). • Hard but brittle. • Many are soluble in water. • DO NOT conduct electricity in the solid state since ions cannot move. • DO conduct electricity in the liquid or aqueous states since charged ions are mobile.


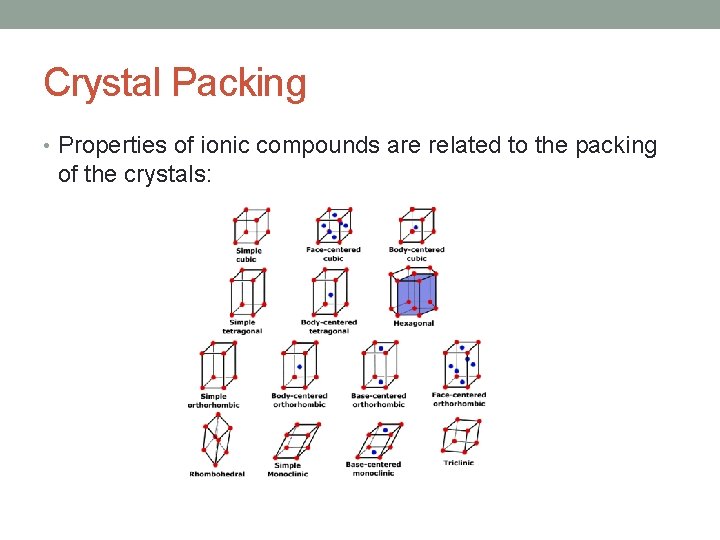
Crystal Packing • Properties of ionic compounds are related to the packing of the crystals:

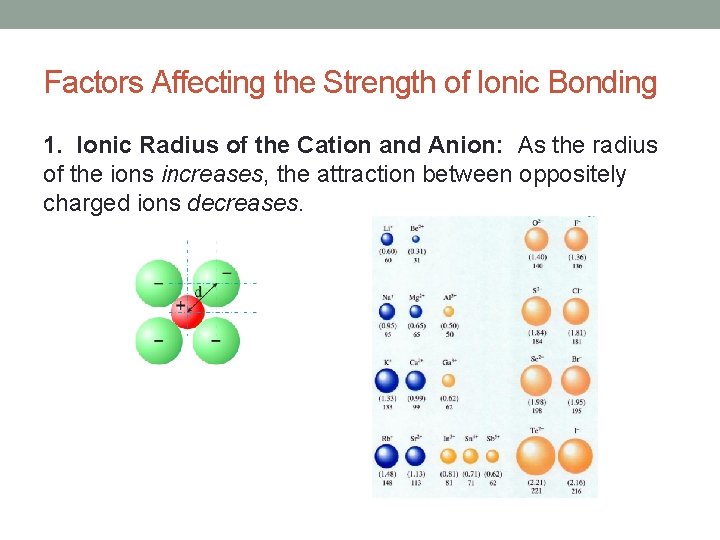
Factors Affecting the Strength of Ionic Bonding 1. Ionic Radius of the Cation and Anion: As the radius of the ions increases, the attraction between oppositely charged ions decreases.

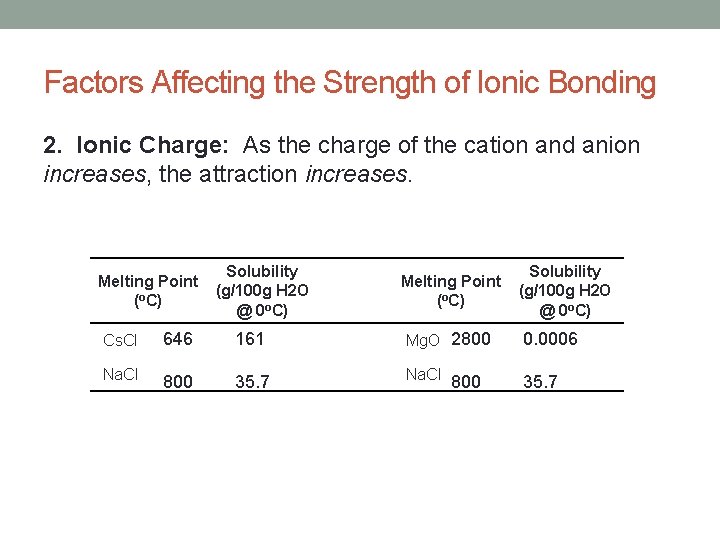
Factors Affecting the Strength of Ionic Bonding 2. Ionic Charge: As the charge of the cation and anion increases, the attraction increases. Solubility Melting Point (g/100 g H 2 O (o. C) @ 0 o. C) Cs. Cl 646 161 Mg. O 2800 0. 0006 Na. Cl 800 35. 7 Na. Cl 35. 7 800

7. Covalent Network Solids • Form a lattice of continuous covalent / polar-covalent bonds. • Do not contain molecules. • Very hard, brittle substances. • Most do not conduct since electrons are either in sigma bonds or lone pairs (filled orbitals). • Some exist as different allotropes (forms with different properties)

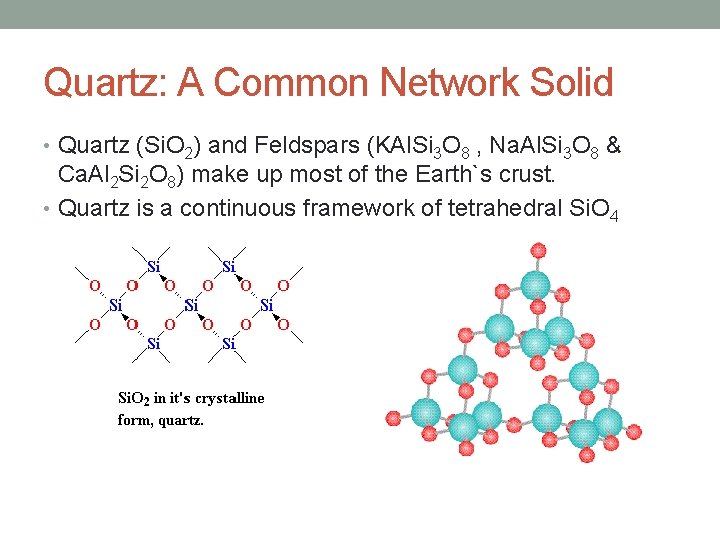
Quartz: A Common Network Solid • Quartz (Si. O 2) and Feldspars (KAl. Si 3 O 8 , Na. Al. Si 3 O 8 & Ca. Al 2 Si 2 O 8) make up most of the Earth`s crust. • Quartz is a continuous framework of tetrahedral Si. O 4

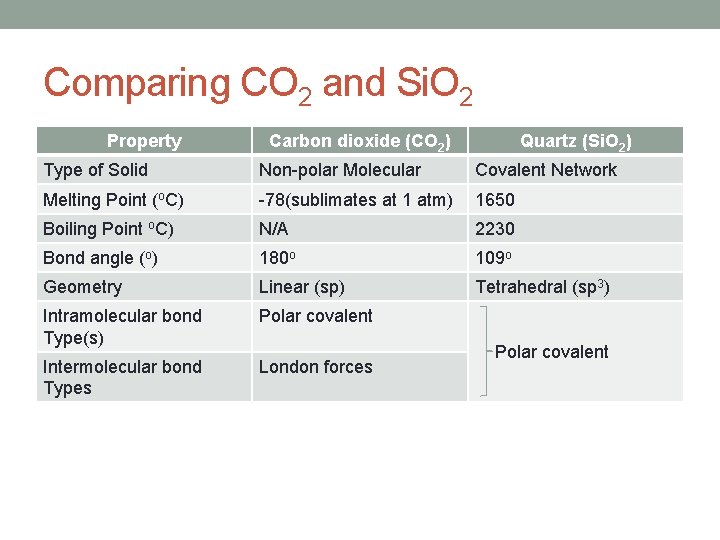
Comparing CO 2 and Si. O 2 Property Carbon dioxide (CO 2) Quartz (Si. O 2) Type of Solid Non-polar Molecular Covalent Network Melting Point (o. C) -78(sublimates at 1 atm) 1650 Boiling Point o. C) N/A 2230 Bond angle (o) 180 o 109 o Geometry Linear (sp) Tetrahedral (sp 3) Intramolecular bond Type(s) Polar covalent Intermolecular bond Types London forces Polar covalent

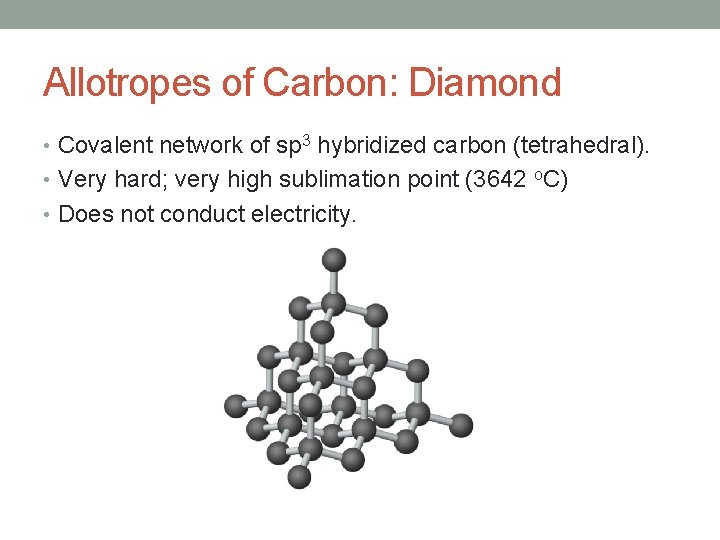
Allotropes of Carbon: Diamond • Covalent network of sp 3 hybridized carbon (tetrahedral). • Very hard; very high sublimation point (3642 o. C) • Does not conduct electricity.

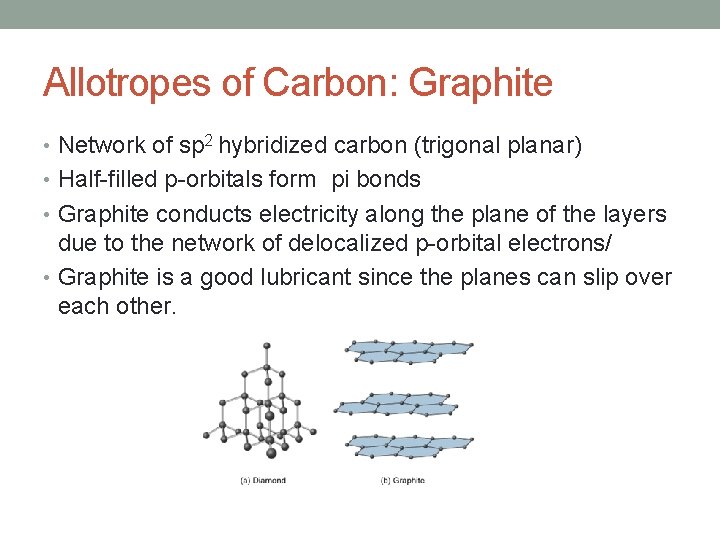
Allotropes of Carbon: Graphite • Network of sp 2 hybridized carbon (trigonal planar) • Half-filled p-orbitals form pi bonds • Graphite conducts electricity along the plane of the layers due to the network of delocalized p-orbital electrons/ • Graphite is a good lubricant since the planes can slip over each other.

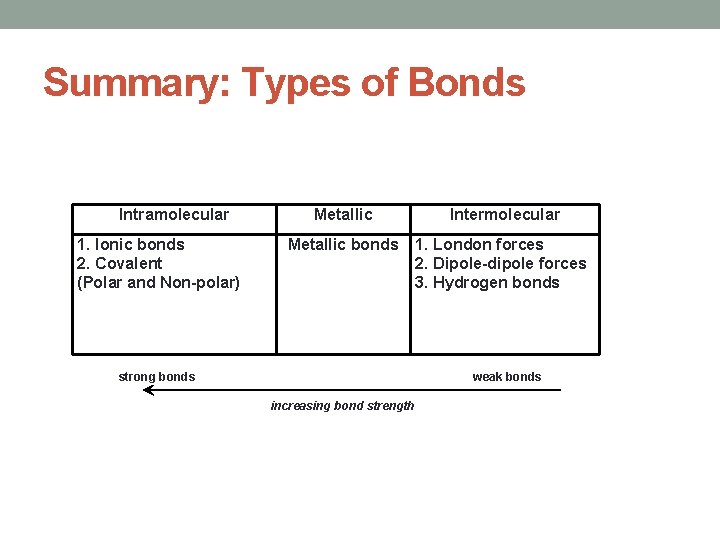
Summary: Types of Bonds Intramolecular 1. Ionic bonds 2. Covalent (Polar and Non-polar) Metallic Intermolecular Metallic bonds 1. London forces 2. Dipole-dipole forces 3. Hydrogen bonds strong bonds weak bonds increasing bond strength

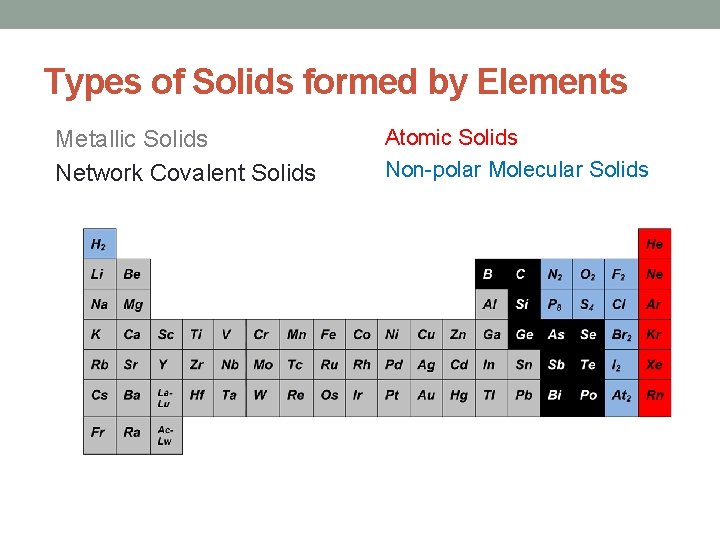
Types of Solids formed by Elements Metallic Solids Network Covalent Solids Atomic Solids Non-polar Molecular Solids

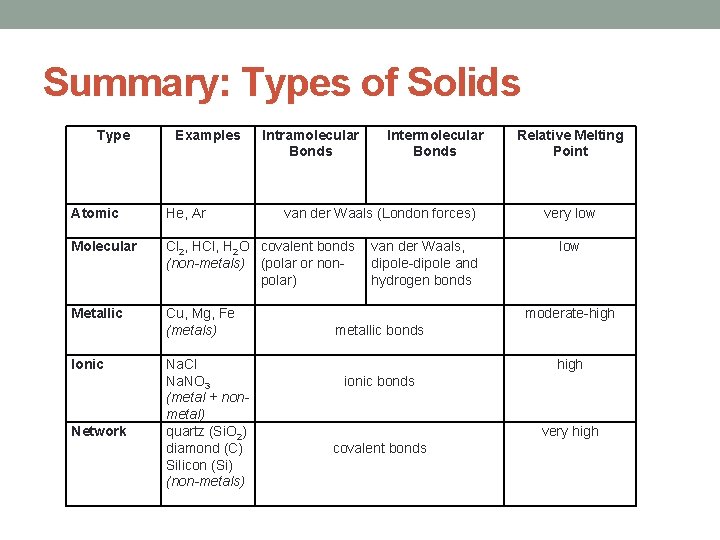
Summary: Types of Solids Type Atomic Molecular Metallic Ionic Network Examples He, Ar Intramolecular Bonds Intermolecular Bonds van der Waals (London forces) Cl 2, HCl, H 2 O covalent bonds van der Waals, (non-metals) (polar or nondipole-dipole and polar) hydrogen bonds Cu, Mg, Fe (metals) metallic bonds Na. Cl Na. NO 3 ionic bonds (metal + nonmetal) quartz (Si. O 2) diamond (C) covalent bonds Silicon (Si) (non-metals) Relative Melting Point very low moderate-high very high
 Viewgrade 5 sch
Viewgrade 5 sch Properties of solids and liquids
Properties of solids and liquids Covalent molecular and covalent network
Covalent molecular and covalent network Properties of solids liquids and gases
Properties of solids liquids and gases Characteristics of platonic solids
Characteristics of platonic solids Elastic properties of solids
Elastic properties of solids Bulk properties of solids
Bulk properties of solids Properties of solids liquids and gases with examples
Properties of solids liquids and gases with examples Superplasticity
Superplasticity Buoyancyability
Buoyancyability Strength property of matter
Strength property of matter How to calculate volume
How to calculate volume Physical and chemical properties
Physical and chemical properties Intensive and extensive properties
Intensive and extensive properties Intrapleural pressure vs intrapulmonary pressure
Intrapleural pressure vs intrapulmonary pressure Myslo.intra
Myslo.intra Intra-elite schism
Intra-elite schism Coxapati
Coxapati Emociones intrapersonales
Emociones intrapersonales Sedu intra
Sedu intra Tige pituitaire
Tige pituitaire Intra thoracique
Intra thoracique Holtech
Holtech Intra entity transactions
Intra entity transactions Exophtalmie grade 2
Exophtalmie grade 2 Intra uems
Intra uems Agencement intra arcade
Agencement intra arcade Woonpremies gent
Woonpremies gent Difference between interpersonal and intrapersonal
Difference between interpersonal and intrapersonal Modello intra 13
Modello intra 13 Displaced intra articular fracture
Displaced intra articular fracture Dsden28
Dsden28 Intra-generational interaction
Intra-generational interaction Inter intra extra
Inter intra extra Intra alveolar pressure
Intra alveolar pressure Intra lase
Intra lase Forme des arcades dentaires
Forme des arcades dentaires Major intra and extracellular electrolytes
Major intra and extracellular electrolytes Variabel sekunder adalah
Variabel sekunder adalah Llums.intra.bt.com
Llums.intra.bt.com Intra siunsote
Intra siunsote